Investigating the Effect of Catalyst Type and Concentration on the functional Group Conversion in Ca
Vol.06No.02(2016), Article ID:65818,11 pages
10.4236/aces.2016.62020
Chigozie F. Uzoh*, Joseph T. Nwabanne
Chemical Engineering Department, Faculty of Engineering Nnamdi Azikiwe University, Awka, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 November 2015; accepted 22 April 2016; published 25 April 2016
ABSTRACT
Significant scientific and economic benefits may be derived from investigating the best choice of catalyst in the alkyd resin synthesis. The effect of catalyst type and concentration on the production of alkyd resin using castor seed oil (CSO) was evaluated. Lithium hydroxide, lead (II) oxide, calcium carbonate, sodium hydroxide and calcium oxide were investigated. The fatty acid profile of the raw CSO was determined using GC-MS while structural elucidation of the CSO based alkyd resins was determined using FTIR spectrometry. The CSO modified alkyd resin produced has acid values of 5.0, 5.61, 7.0 8.24 and 11 for lithium hydroxide, lead (II) oxide, calcium carbonate, sodium hydroxide and calcium oxide respectively. The extent of reaction was 95%, 95%, 91%, 89% and 88% for lithium hydroxide, lead (II) oxide, calcium carbonate, sodium hydroxide and calcium oxide respectively at the reaction time of 150 minutes. The alcoholysis reaction completion time was fastest in LiOH followed by PbO, CaCO3, NaOH and CaO catalyst. Physico-chemical parameters of the oil and performance evaluation of the alkyd films suggest that they are sustainable materials for surface coating. LiOH shows excellent robustness to expanded process parameters.
Keywords:
Alcoholysis, Alkyd Resin, Dehydrated Castor Seed Oil, Catalyst Type, GC-MS

1. Introduction
Scarcity of fossil resources, fear of depletion of the reserve and fluctuation in their prices is responsible for the uncertainty in the future availability and supply of petroleum products as the major source of raw materials in different industrial fields [1] . Thus, there has been an increased interest in the search and development of renewable resources that would stand out as valuable alternative.
However, despite the apparent popularity of petroleum products as raw materials in different areas of application, fats and oils are being greatly favoured for use in surface coatings, soaps, cosmetics, pharmaceuticals, lubricants, surfactants and polymer processing. Their wide acceptance in these fields of applications is attributable to their being renewable resources, sustainability, biodegradability, and environmental friendliness which are becoming more significant considerations in recent times due to the pressing environmental challenges of climate change and global warming. This development has given rise to a number of investigations on the quality and applications of vast number of African seed oils. It has also triggered the demand for more oils needed to expand the present supplies in the oleo-chemical industry [1] .
The oils used by chemical industries as raw materials for production can be obtained from plants, animals and from marine life. Although most oils are obtained from plants (60%), a sizable quantity (36%) comes from animal sources and the rest (4%) from marine life. Examples of animal fats and oils are fallow, lard, butter and cod liver oil [1] . These animals and marine fats and oils are used extensively in industries. Hence, growth of chemical industries has stimulated a simultaneous expansion of the use of fats and oils as raw materials and also as intermediate for sources of new chemicals. Alkyd resin is one of such products from oils used extensively in industries.
Alkyd resins are any large group of thermoplastic resins that are essentially polyester products formed from the polymeric condensation of polyhydric alcohol, polybasic acid and monobasic fatty acids. They are one of the major raw materials used in the formulation of surface coatings. Although other surface coating materials like latex resins and powder coatings were more recently discovered; alkyd resins still remain a mainstay for a number of applications due to their balance of economies and performance. It has been reported that alkyd resins contribute about 70% to the conventional binders used in coating industry today. They contribute to coating flexibility, adhesion, durability, and gloss. The popularity of alkyd resins as vehicle for coatings is largely due to their unique properties such as film hardness, durability gloss and gloss retention, resistance to abrasion, etc. im- pacted on them through modification with drying oil [2] .
The oils that are mostly employed for alkyd resin synthesis are linseed, soybean, castor and tall oils [3] - [5] . These oils are largely imported to Nigeria for the formulation of coatings for metal cans used in packing of beverages, drugs, food, etc. It therefore remains imperative for a search of a suitable local alternative to foreign resins, which will definitely reduce the cost of imported ones.
Castor plant, Ricinus communis L. is a species of flowering plant in the spurge family; Euphorbiaceae, which contains a vast number of plants mostly native to the tropics [6] . Castor oil is derived from the seed of castor plants. They are typically composed of triglyceride molecules (technically called esters) which contain a 3- carbon alcohol (glycerol) and three 18-carbons (or 16-carbons) fatty acids. Castor oil is unique among vegetable oils because it is the only commercial source of a hydroxylated fatty acid (ricinoleic acid). The oil contains around 90% of the fatty acid [7] . Castor oil has been valued as semi-drying oil and has lubricating and hydraulic properties. Modification of castor oil by increasing the unsaturation resulted in an oil with excellent drying properties for use in the production of alkyd resin [8] . Its high conjugated, linoleic properties, which aids rapid cross-linking at elevated temperatures make it ideal for use as a close substitute for linseed oil. The use of castor oil as raw materials for surface coatings application is its ability to polymerize or “dry” after they have been applied to a surface to form tough, adherent, impervious, and abrasion resistance films. The advantages found in surface coating applications include excellent odor, good drying properties, more uniform polymer structure, and lack of after-yellowing. The desaturated castor oil is non-yellowing oil and so can meet requirements of coating industries [9] - [13] . Dehydrated castor oil (DCO) has been found to be a sustainable material for alkyd resin synthesis in terms of its availability, biodegradability and renewability. Dehydrated castor oil modified alkyd resin has been synthesized using two criteria; the resin met all the technical and industrial standards of durability, short drying time, resistance to chemical, etc., and, met all the ecologically relevant standards [14] - [16] .
The demand for alkyd resins has increased over the years even though technical information on local production is scanty. Critical review of literature indicates that the influence catalyst type is basically lacking. Ibanga et al., [17] and Aydin et al., [18] reported the effect of anhydride type and concentration on the viscosity and film properties of cotton seed oil and sunflower oil respectively. They concluded that maleic anhydride can substitute phthalic anhydride in alkyd synthesis without compromising those properties that will lead to quality product. Therefore, a lot of scientific and economic benefit may be achieved from the evaluation of the effect of catalyst in the alkyd resin synthesis. In this research therefore, the effect of catalyst type and concentration on dehydrated castor oil-based resin were examined.
2. Materials and Methods
2.1. Materials
The castor seeds (shown in Figure 1) were purchased from Achi in Orji River, Enugu State. The seed were dehulled to obtain the kernels. The kernels were sun dried for one week. The dried kernels were milled and the oil extracted by the solvent method using a soxhlet extractor. A rotary evaporator equipped with digital thermometer, rotatable round bottom flask, condenser and water were also used. Other equipment include; electronic weighing balance, heating mantle, magnetic stirrer and general laboratory glassware.
Phthalic anhydride (C6H4(CO)2O) with minimum assay > 97%, glycerol (C3H8O3) with assay > 99%, sodium bisulphate (NaHSO4) with assay 97.5%, lithium hydroxide (LiOH), calcium carbonate (CaCO3) and Lead(II) Oxide (PbO) with assay > 96.8% were purchased from BDH chemicals ltd, Poole, England. Diethyl ether, ethanol, xylene, anhydrous methanol,calcium oxide (CaO), sodium hydroxide (NaOH) with assay 96%, were supplied by wharefedale laboratories, Yorkshire, England. The chemicals purchased were of highest purity.
2.2. Methods
2.2.1. Refining of Castor Seed Oil (CSO)
The refining of the crude Castor seed oil (CSO) was carried out according to AOCS standard [19] . 200 g of the raw oil was heated in a flask to 60˚C then, a strong 2N NaOH was added to neutralize the free fatty acid (FFA) content at constant stirring. The chemical reaction involved in this process is as follows;
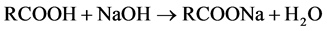
The progress the neutralization reaction was monitored using phenolphthalein indicator. Towards the completion of the neutralization process, NaOH was added to the mixture to ensure adequate salting or graining out the soap stock produced. The mixture was later poured into separating funnel. At about three hours thereafter, the mixture separated into two different layers. The lower layer which contains the FFA rich soap stock was drained out while the upper layer containing the neutralized oil was then washed with hot water to remove any traces of soap. The washing was continued until color of phenolphthalein indicator did not change to pink.
The neutralized washed oil was introduced in a cylindrical vessel equipped with agitator called “Bleacher” and then heated up to 90˚C with steam under vacuum pressure. The moisture from oil was evaporated and oil becomes dry. The dried oil was treated with 5% by mass of bleaching agent (mixture of fuller’s earth and carbon at 1:1 ratio) to improve the adsorption of the residual color. The process was allowed to progress for about 45 minutes with continuous agitation. After which the resulting mixture was filtered through a standard plate and frame press to enhance separation to obtain clear oil very much lighter in color than the neutralized oil. The oil charge was dehydrated under vacuum to avoid any further deterioration due to oxygen. The bleached dehydrated oil was weighed to calculate oil loss and the FFA content was determined. The characteristic of the crude oil and neutralized oil were determined according to AOCS standard [19] .

Figure 1. Castor seed.
2.2.2. Synthesis of the Alkyd Resin
CSO modified alkyd resin was prepared with the dehydrated oil, glycerol and phthalic anhydride using LiOH, CaO, CaCO3, PbO and NaOH as catalyst (not as the same time). The reactions were carried out in a three necked round bottom flask titled with a motorized stirrer, a dean-stark trap titled with water-cooled condenser and nitrogen in let tube at a temperature of 140˚C - 240˚C. Xylene was employed as an azeotropic solvent. During alcoholysis, the triglycerides of CSO were converted to monoglycerides by reaction with glycerol at 140˚C - 240˚C. PA, glycerol and xylene were then added, and polymerization was carried out at 200˚C - 240˚C for more than 6 h as shown in Figure 2.
The concentrations of alkyd solutions were determined and finally diluted to 70% - 75% by addition of xylene. The progress of reaction was followed by measuring the evolved water of reaction and periodically checking the acid number by titration. The first stage was alcoholysis and the second stage was esterification.
Alcoholysis: Monoglyceride was first synthesized by reacting the oil with glycerol. Alcoholysis of oil was carried out with 0.1% (by wt) all the catalyst (LiOH, CaO, CaCO3, PbO and NaOH). In alcoholysis reaction, the oil was heated with agitation speed of 700 rpm to 180˚C. Glycerol and selected catalyst were added and alcoholysis reaction occurs at 140˚C - 220˚C. The reaction was continued until a sample of the reaction mixture became soluble in 2 to 4 volume of anhydrous methanol. After alcoholysis was completed, the reaction mixture was cooled to 140˚C before esterification commence.
Esterification: Phthalic anhydride was added to the monoglyceride mixture. The temperature of mixture was maintained at the range of 230˚C - 240˚C. The reaction progress was monitored by periodic determination of the acid value. The second stage reaction is continued at the elevated temperature and long chain molecules are formed which contain excess hydroxyl group (Figure 3). At this state water is released. Removal of water from the mixture was facilitated by a solvent extraction method. The mixed vapor generated was then condensed and collected. By choosing the exact solvent (xylene) that does not mix with water in liquid phase the mixture demarcated into two layers. Xylene is chosen for this process since it has suitable boiling point and low water solubility. When the acid value dropped to a value somewhat below 8, the reaction is stopped. The acid values of the alkyd resin were determined and chemical resistance was equally measured.

Figure 2. Alcoholysis stage.

Figure 3. Second stage reaction with phthalic anhydride (PA).
2.2.3. Instruments
The analysis of the oil was performed with a Thermo Finnigan Trace GC/Trace DSQ/A1300, (E.I Quadropole) equipped with a SGE-BPX5 MS fused silica capillary column (film thickness 0.25 lm) for GC?MS detection, and an electron ionization system with ionization energy of 700 eV was used. Carrier gas was helium at a flow rate of 10 ml∙min−1 injector and MS transfer line temperatures were set at 220 and 290˚C, respectively. The oven temperature was programmed from 50 to 150˚C at 3˚C∙min−1, then held isothermal for 100 min, and raised to 250˚C at 10˚C∙min−1. Diluted samples (1/100, v/v, in methylene chloride) of 1.00 l L were injected manually in the slitless mode. The identification of individual components was based on the comparison of their relative retention times with those of authentic samples on SGE-BPX5 capillary column, and by matching their mass spectral of peaks with those obtained from authentic samples and/or the Wiley 7N and TRLIB libraries spectra and published data. The chemical compositions of oil were also confirmed by SHIMADZU FTIR-84008. Viscosity was determined by Brookfield viscometer, RVT Model (#Spindle 3, RPM 20). The physico-chemical properties of the raw and modified oil were determined by standard methods (ASTM, 1973).
2.3. Performance Evaluation
The drying performance, film characteristics, chemical properties of the alkyds prepared were determined by standard methods [20] .
3. Results and Discussion
3.1. Characterisation of Castor Oil Samples
The physiochemical properties of the castor seed oil is shown in Table 1. It had a specific gravity of 0.9125 which indicated that the oil is less dense than water. The iodine value was 93.15 which imply that castor oil is a semi-drying oil [21] . Iodine value is a vital parameter employed in ascertaining the suitability of oil for alkyd synthesis. It shows the level of unsaturation of the oil. The acid value of castor seed oil was 13.465 mg NaOH/g, as it was used to measure the level of deterioration of oil. Sometimes high acid value of oil could be due to hydrolytic reaction during processing of the oil or as a result of enzymatic action in the oil bearing seed [22] . The saponification value of castor seed oil was 179.95 mg KOH/g was within acceptable limits [23] . The saponification value reveals average molecular weight of fatty acids of trigyceride present in castor seed oil. Even though, the specific gravity and iodine value of crude oil were in the range of ASTM specification limit, the free fatty acid content of crude oil differed from ASTM standard. To reduce the free fatty acid content of the oils essentially involves neutralization of the crude oil samples. High free fatty acid content in the oil increases the curing or drying time of the resin. In neutralization process, it was found that 60 ml of 2N NaOH per 200 g of oil was required to obtain neutralized oil with a lower free fatty acid content. The neutralization of 20 minutes was sufficient to reduce the free fatty acid content of castor oil from 6.732 to 2.2. In neutralization of oil, the free fatty acid content of oil was converted in oil soluble soaps. Small amount of impurities such as phosphatides, proteins or protein fragments, and gummy or mucilaginous substances were also removed by neutralization process. In
Table 1. Characteristics of the castor oil samples.
neutralization process, it was difficult to separate the soap and oil layer because free fatty acid content in crude oil was very high. The two layers were easily separated when NaCl solution was added to the neutralized mixture because NaCl help to ensure adequate salting or graining out of the soap stocks. Other impurities in oil were removed by washing with hot water. From Table 1, there is an increase in the iodine value from 95.20.20 to 135.95 and acid value 4.45 to 6.7 which makes the castor oil a drying oil according to ASTM standard of dehydrated castor oil. The viscosity value and the specific gravity value were also in the limit of ASTM standard of dehydrated castor oil. Therefore, the dehydrated castor oil is acceptable for alkyd resin synthesis.
3.2. Synthesis of Alkyd Resin
According to Ikhuoria et al. [24] , the alcoholysis reaction is usually completed within an hour or two hours after the batch had reached operating temperature. Table 2 show the solubility of the alcoholysis samples in anhydrous methanol carried out for several hours using NaOH, PbO, CaO, CaCO3, LiOH as catalysts. In the esterification reaction, it was observed that the longer the reaction, the more viscous the mixture becomes. In this stage, adequate agitation was necessary for complete mixing of monoglyceride mixture and phthalic anhydride. It was observed from the table that most of the samples of the alcoholysis mixture did not completely dissolve in anhydrous methanol after four hours for alcoholysis reactions carried out using 0.1 and 0.2 wt% CaO, 0.1 and 0.2 wt% NaOH, 0.1 wt% PbO and 0.1 wt% CaCO3. It was also observed that as the catalyst weight percentage increased for each catalyst, the time required for the completion of the alcoholysis reaction decreased. From Table 2, it was observed that the reaction using LiOH in the alcoholysis stage proceed with fastest time followed by PbO, CaCO3, NaOH and CaO in that order which shows that LiOH is most suitable for the synthesis of alkyd resin using modified castor seed oil.
3.3. Physiochemical Characteristics of Alkyd Resin
In Table 3, there is no common standard to compare alkyds resins. Each alkyd resin has its own properties. The alkyd resin that has acid number of less than 15 is suitable for application of paint, and all the alkyd samples produced were at acid value below 15 mg NaOH g−1 [23] [25] [26] . This is because a higher acid value translates to reduced drying rate, since acid group usually delay drying rate [27] . In addition, industrial products (such as paints) formulated with alkyd resin of high acid value usually cause rusting or corrosion of substrate surfaces. Hence, such alkyds are associated with poor performance characteristics. The darkening colour of the alkyd could be attributed to the high temperatures of reaction, oxidation and catalyst. This alkyd can be utilized in the production of pigmented coatings where very bright colour is not a major requirement. The iodine values of the alkyds were observed to have decreased considerably for all alkyd resin produced using the different catalyst as compared to that of castor seed oil. The iodine value points at the level of unsaturation, thus it affects the drying qualities. The decrease in level of unsaturation of the alkyds could be attributed to the dimerization and polymerization reactions at the reactive double bonds of the oil during alkyds synthesis [28] . The saponification values of the alkyds resin was observed to be lower in LiOH followed by PbO, CaCO3, NaOH and CaO respectively.
3.4. Chemical Resistance of Alkyd Resin Film
The resistance of alkyd resin film was determined in two media, distilled water and NaOH solution. Table 4 described that there was no effect on alkyd film after immersion in distilled water for 24 hours. The immersion of alkyd film in water for 24 hours was sufficient time to examine the water resistance. When the alkyd film was immersed in strong alkali solution, 3N NaOH, the film got whitening after immersion time for 7 hours, blistering after immersion time for 15 hours and removal after immersion time for 24 hours, this shows that they are resistant to brine, water and acid.
3.5. Variation of Acid Value with Reaction Time
The variation of acid value with reaction time is shown in Figure 4. It was observed that there was a sharp decrease which slowed down at later stages in the reaction. This was attributed to the different reactivities of primary-OH and secondary-OH groups of the glycerol with carboxyl group of the phthalic anhydride as reported in literature [29] . Thus, during the initial rapid decrease in the acid value for all the samples corresponded to the
Table 2. First stage alcoholysis reaction.
Table 3. Characteristics castor oil modified alkyd resin.
Table 4. Chemical resistances of alkyd resin film.

Figure 4. Variation of acid value with reaction time.
reaction of the primary-OH of the monoglyceride while the other portion of the plot where reaction was slow corresponded to the reaction of the secondary-OH. It was also observed that the rate of decrease in acid value as the reaction proceeds was greatest in LiOH, followed by PbO, CaCO3, NaOH and CaO respectively. From Figure 4, it was observed that at a particular reaction time, the sample with the least acid value was that being produced by LiOH followed by PbO, CaCO3, NaOH and CaO which makes LiOH the best catalyst suitable for alkyd resin synthesis.
3.6. Variation of Degree of Polymerization (DP) with Reaction Time
The graphical variation of degree of polymerisation with time is presented in Figure 5. It is expected that the graphs should be linear throughout the reaction. However, Figure 5, shows that the DP increase linearly at the early stage of the reaction up to the gel point (i.e. the point at which network formation commenced), where deviation from linearity occurs. The initial linear portion corresponds to a period where the primary hydroxyl (α-OH) group of glycerol reacted rapidly with carboxyl group of phthalic anhydride to form linear molecules [29] . This point of commencement of deviation from linearity indicates the portion at which the secondary hydroxyl (β-OH) group (which is less reactive than α-OH) reacts with the phthalic acid to form three dimensional molecules [30] . This region is considered to mark changes in physical characteristics (such as viscosity) of the reaction. Figure 5 also shows that LiOH and PbO alkyd resin attained the highest degree of polymerization at the shortest possible time.
3.7. Extent of Reaction with Reaction Time
The extent or fraction of reaction is defined as the fraction of the hydroxyl or carboxyl functional group that has reacted at a given time. From Figure 6, the extent of reaction was found to be increase as the reaction time increases for all the different catalysts used. The reaction with LiOH and PbO catalyst attained the highest extent of reaction followed by CaCO3, NaOH and CaO catalyst at the same reaction time as can be seen in Figure 6.
3.8. FTIR and GC-MS Analysis of Castor Oil-Modified Alkyd Resin
The FTIR spectra of the castor oil modified alkyd resin are described as given. All spectra have common peaks because the structures of alkyd resin are very similar. At 3600 cm−1 shows the presence of hydroxyl (O-H) of the unsaturated fatty acid in castor oil. The absorption band at 3000 cm−1 is a characteristics of alkenes (=C-H) group [31] [32] . The alkyd resin exhibits a characteristics of straight chain ester band (O=C-O-C) at 1167.69 cm−1. The appearance of straight chain of alkaneCH2, -CH- is found at 2940 cm−1 and 1495 cm−1. The peak at 1308.54 cm−1 shows methyl bending (-CH3). Finally, the band at 1000 cm−1 corresponds to stretching of ether (-C-O-C). In Table 5, component C16:2 (palmitoleate) with value of 84.4856 ppm show the highest concentra-

Figure 5. Variation of degree of polymerization with time.

Figure 6. Variation of extent of reaction with time.
Table 5. GC-MS analysis for castor oil.
tion of the alkyd resin produced, C18 (methyl stearate) has a value of 37.6286 ppm follow by C16 (palmitic acid), C14 (myristse), C20 (Behenic acid), C17 (Magaric acid) and C12 (Lauric acid) which show the lowest concentration of 1.2072 ppm.
4. Conclusion
The effect of different catalyst on alkyd resin production was carried out using castor to determine the most suitable catalyst for its production. It was observed from the result analysed that the rate of decrease in acid value, extent of reaction, degree of polymerization was higher in LiOH and PbO followed by CaCO3, NaOH and CaO. The physiochemical properties of the alkyd resin production using 0.3 wt% of all the catalyst also shows that LiOH and PbO produced better quality alkyd resin followed by CaCO3, NaOH and CaO. Therefore, it can be concluded that LiOH and PbO are the most suitable catalyst used for alkyd resin synthesis.
Cite this paper
Chigozie F. Uzoh,Joseph T. Nwabanne, (2016) Investigating the Effect of Catalyst Type and Concentration on the functional Group Conversion in Castor Seed Oil Alkyd Resin Production. Advances in Chemical Engineering and Science,06,190-200. doi: 10.4236/aces.2016.62020
References
- 1. Asiagwu, A.K., Omuku, P.E., Okoye, P.A.C., Olisa, M.A. and Ajiwe, V.C.E. (2008) Evaluation of the Suitability of Conophor Oil for the Production of Alkyd Resins and Surface Coatings. Oriental Journal of Chemistry, 24, 405-408.
- 2. Aigbodion, A.I. and Okieimen, F.E. (2001) An Investigation on the Utilization of African Locust Bean Seed Oil in the Preparation of Alkyd Resins. Industrial Crop and Products, 13, 29-34.
http://dx.doi.org/10.1016/S0926-6690(00)00050-9 - 3. Ogunniyi, D.S. and Njikang, G.N. (2000) Preparation and Evaluation of Alkyd Resins from Castor Oil. Pakistan Journal of Scientific and Industrial Research, 42, 378-380.
- 4. Kildiran, G., Yucek, S.O. and Turkay, S. (1996) In-Situ Alcoholysis of Soyabean Oil. Journal of American Oil Chemical Society, 73, 252-812.
http://dx.doi.org/10.1007/BF02523899 - 5. Majumder, S., Kumar, D. and Nirvan, Y.P.S. (1999) Acrylate Grafted Dehydrated Castor Oil Alkyd. A Binder for Exterior Paints. Paint India, 60, 57-65.
- 6. Moshkin, V.A. (1986) Castor Amerind. New Delhi.
- 7. Ogunniyi, D.S. (2006) Castor Oil: A Vital Industrial Raw Material. Bioresource Technology, 97, 1086-1091.
http://dx.doi.org/10.1016/j.biortech.2005.03.028 - 8. Spyros, A.J. (2004) Characterization of Unsaturated Polyester and Alkyd Resins Using One- and Two-Dimensional NMR Spectroscopy. Journal of Applied Polymer Science, 88, 1881-1888.
http://dx.doi.org/10.1002/app.11950 - 9. Waters, R.T. (1955) Resins-Synthetic, Alkyd Resins, Section 2. Wyman and Sons, Ltd., London.
- 10. Formo, M.W., et al. (1965) Bailey’s Industrial Fats and Oils Products. Vol. 1. 4th Edition. John Wiley and Sons, Inc., New York.
- 11. Kirk, R.F. and Othmer, D.F. (1947) Alkyd Resin: Encyclopedia of Chemical Technology. Vol. 9, John Wiley and Sons, Inc., New York.
- 12. Mark, H.F. (1964) Alkyd Resin: Encyclopedia of Polymer Science and Technology. Vol. 1, Interscience Publishers, a Division of John Wiley and Sons, Inc., New York.
- 13. Mark, H., Proskauer, E.S. and Frilette, V.J. (1954) Resins, Rubbers, Plastics Yearbook. Interscience Publishers, a Division of John Wiley and Sons, Inc., New York.
- 14. Onukwuli, O.D. and Igbokwe, P.K. (2008) Production and Characterization of Castor Oil-Modified Alkyd Resins. Journal of Engineering and Applied Science, 2, 161-165.
- 15. Hlaing, N.N. and Mya, O. (2008) Manufacture of Alkyd Resin from Castor Oil. World Academy of Science and Engineering and Technology, 24, 115-161.
- 16. 23 April 2014.
http://www.dainet.de/fnr/ctvo/paint/2_workshop/ull.doc - 17. Ibanga, O.I. and Edet, W.N. (2013) Influence of Polybasic Acid Type on the Physicochemical and Viscosity Properties of Cotton Seed Oil Alkyd Resins. International Journal of Engineering and Sciences, 2, 1-14.
- 18. Aydin, S., Akcay, H., Ozkan, E., Guner, S. and Erciyes, T. (2004) The Effects of Anhydride Type and Amount on Viscosity and Film Properties of Alkyd Resin. Progress in Organ Coating, 51, 273-279.
http://dx.doi.org/10.1016/j.porgcoat.2004.07.009 - 19. AOCS (1998) Official Methods and Recommended Practices of the American Oil Chemist’s Society. 4th Edition, AOCS, Champaign.
- 20. Kirk-Othmer (1966) Kirk-Othmer Encyclopedia of Chemical Technology. 2nd Edition, Vol. 1, Interscience Publishers, New York, 851-882.
- 21. Wicks, Z.W., Jones, N.F. and Papas, S.P. (2007) Organic Coatings Science and Technology. 3rd Edition, John Wiley & Sons Inc., Hoboken.
http://dx.doi.org/10.1002/047007907X - 22. Cocks, V.L. and Rede, C.V. (1966) Laboratory Handbook for Oil and Fats Analysis. Academic Press, London.
- 23. Patton, T.C. (1962) Alkyd Resin Technology. Formulating Techniques. Interscience Publication, New York.
- 24. Ikhuoria, E.U., Aigbodion, A.I. and Okieimen, F.E. (2004) Enhancing the Quality of Alkyd Resins Using Methyl Ester of Rubber Seed Oil. Tropical Journal of Pharmaceutical Research, 3, 311-317.
- 25. Mark, H., Proskauer, E.F. and Frilette, V.I. (1954) Resins, Rubbers Plastic Yearbook. Interscience Publishers, Inc. A Division of John Wiley and Sons, Inc., New York.
- 26. Mehlenbacher, V.C. (1950) Official and Tentative Methods for the American Oil Chemists’ Society. Second Edition, American Oil Chemists’ Society (AOCS), Chicago.
- 27. Hymore, F.K. and Audu, T.O.K. (1991) Studies on the Drying of a Rubber Seed Oil Based Medium Alkyd Resin.
- 28. Momodu, V.M., Omorogbe, S.O., Ikhuoria, E.U. and Aigbodion, A.I. (2011) Synthesis and Evaluation of Performance Characteristics of Walnut (Tetracarpidium conophorum) Seed Oil-Modified Alkyd Resin. Researcher, 3, 63-66.
- 29. Aigbodion, A.I. and Okieimen, F.E. (1991) Kinetics of the Preparation of Rubber Seed Oil Alkyd. European Polymer Journal, 32, 1105-1108.
http://dx.doi.org/10.1016/0014-3057(96)00053-5 - 30. Yahaya, L.E., Aigbodion, A.I. and Bakare, J.O. (2001) Investigation of Network Formation in Rubber Seed Oil Alkyd System by Dilute Solution Viscometry. Nigerian Journal of Science, 35, 117-122.
- 31. Grasselli, J.G., Mocadlo, S.E. and Mooney, J.R. (2000) Applied Polymer Analysis and Characterization, Analysis of Polymer by Fourier Transform Infrared Spectrometry. Vol. 2, Oxford University Press, New York.
- 32. Sanler, S.R. (1998) Polymer Synthesis and Characterization: A Laboratory Manual. Academic Press, a Division of Harcourt Brace and Company, San Diego.
NOTES

*Corresponding author.
上一篇:Solubility of Solids in Superc 下一篇:Synthesis and Polymerization o
最新文章NEWS
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Solubility of Solids in Supercritical Fluids: The Mendez-Santiago Teja Model Revisited
- Solventless Organic Reactive Crystallization at Mild Conditions
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Luminescent Characteristic of Organic Compound-Containing Inorganic Crystal at Room Temperature
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Tunable Polymorphic Transformation Temperature
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- A Convective Thin Layer Drying Model with Shrinkage for Kent Mango Slices
- Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Optimisation of Decolourisation Conditions of Crude Shea (Vitellaria paradoxa Gaertner F) Butter: Ye
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Solvent Extraction of Citric Acid with Different Organic Phases

