Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
Vol.05No.04(2015), Article ID:59062,4 pages
10.4236/aces.2015.54043
Liangmou Yu1, Bo Shu2, Shiwen Yao2
1Faculty of Environmental Engineering, Kunming Metallurgy College, Kunming, China
2Yunnan Copper Co., Ltd., Kunming, China
Email: xiashubiao401@163.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 July 2015; accepted 22 August 2015; published 25 August 2015
ABSTRACT
In this work, we recover cobalt from waste 18650-type lithium-ion batteries by acid leaching. The cathode material is completely dissolved, after leaching waste batteries by using 10 mol/L industrial sulfuric acid at 70˚C for 1 h. The rate of cobalt leaching is nearly 100%. Removal of sodium carbonate, iron, aluminum and other impurities from the leaching solution was well performed by adjusting the pH to 2 - 3 with stirring vigorously. Finally, under the conditions of 55˚C - 60˚C of 240 A/m2 current density, electrodeposition current efficiency was 90.01%, the quality of the electrical output achieved cobalt 1A standard electrolytic cobalt, cobalt until greater than 90% yield. The process is easy and suitable for large-scale lithium-ion batteries used in the recovery of valuable metals.
Keywords:
Component, Cobalt, 18650-Type Lithium-Ion Battery, Leaching, Electrodeposition

1. Introduction
Rechargeable lithium-ion batteries as a new generation of green, non-pollution chemical energy storage device are widely used in portable electronic apparatus and vehicles. However, waste LIBs contain heavy metals, organic chemicals and plastics, which will bring environmental pollution. Therefore, the recycling of major components from spent LIBs is considered to be a beneficial way to prevent environmental pollution and as alternative resource of cobalt [1] . 18650-type power batteries are widely used in small portable devices, increasing along with the rise of electric vehicles in Tesla [2] . The spent lithium ion batteries contain 5 - 15 wt.% cobalt and 2 - 7 wt.% lithium as important constituents, which are an active cathodic material. The waste 18650 type lithium ion batteries contain precious metal elements cobalt with a certain toxicity as important constituents, which are an active cathodic material. Since cobalt is a relatively expensive material compared to the other battery constituents and can be widely used in new LiBs electrode materials, its recovery is one of the primary objectives in the recycling of spent batteries [3] [4] . Figure 1 is a 18650 type battery price polyvalent metallic element [5] . It is not difficult to see from Figure 1 that the price of the cobalt is more expensive than the other battery constituents. Therefore the recovery and utilization of cobalt have significant economic and social environmental benefits [6] .
Separation and Recovery Technology 18650 cobalt lithium ion battery mainly includes three steps: 1) the waste batteries discharge, stripped shell, simple crushing, after screening to obtain an electrode material, or simply crushed after firing to remove organic matter to obtain an electrode material [7] ; 2) the material obtained in the first step of the electrode is dissolved in the leaching of various metals into solution wherein both the cobalt and nickel represent as trivalent forms. Leaching sub-step dissolution method and two-dissolution method: direct step by acid leaching dissolution method, all the metal was dissolved in acid, and then isolated using a number of different purification and recovery; two-step method is to use an alkali leaching aluminum and recovered, and then use acid leaching surplus metal oxide, followed by the similar treatment as the first step [8] ; 3) the solution (leachate) dissolved metal elements in the solution is separated for recycling or direct synthesis anode material. Separation and recovery methods are chemical precipitation, salting out, ion exchange, extraction, electrochemical method, respectively cobalt or lithium-containing compound [9] . E.M.S. Barbieri et al. [10] have investigated recycling cobalt from the cathodes of spent Li-ion batteries as β-Co(OH)2, obtaining Co3O4. β-Co(OH)2 with a hexagonal structure by using chemical precipitation or electrochemical precipitation. This method does not directly recovered cobalt. In this paper, we recovered directly cobalt by electrodeposition.
2. Experimental
Experimental material: Waste 18650 lithium-ion battery, industries concentrated sulfuric acid, industrial grade sodium borate. Hand split waste 18650 lithium-ion batteries, remove the interior material with pliers, and finally the positive and negative material separated and dried 24 h at 60˚C. The obtained positive electrode material with 10 mol/L sulfuric acid leaching 1h, and filtered to give leachate. Spectrophotometric determination of Co2+ concentration in the solution (722S spectrophotometer, Shanghai Precision Scientific Instrument Co., Ltd.) with cobalt ions spectral qualities, using X-ray diffraction (Japan Rigaku company DM/ax-IIIA) salting crop products phase analysis. Process is shown in Figure 2, ICP analysis content of the test solution of various metal elements.
3. Results and Discussion
3.1. Effect of pH on the Leaching Process
When the solution of sulfuric acid concentration is too low, even at boiling temperature, leaching reaction is also extremely slow. Therefore, the leaching temperature was set at 70˚C, under the conditions of the reaction time of 1 h, acidity leaching test, the results shown in Figure 3 shows that, as the solution acidity increase, cobalt and

Figure 1. Price metal elements contained at 18650 lithium-ion batteries.

Figure 2. Process flow diagram of cobalt recovery by liquid leaching.


Figure 3. Effect of acid concentration on leaching rate and electrolytic cobalt photo.
aluminum solubility in the solution increases accordingly. With 10 mol/L sulfuric acid solution as an industrial leaching agent, the lithium-ion battery with the waste placed in 5 L beaker, heated to 70˚C, leaching 1 h, the reaction was completely dissolved, ICP testing the resulting solution composition shown in Table 1.
3.2. Neutralization Reaction to Remove Iron, Aluminum
Leaching with sodium carbonate as a neutralizing agent resulting solution was adjusted to pH 2 - 3, and heated to 90˚C, blast stirred solution of iron and aluminum precipitate. At the same time, also co-precipitation in the form of silicon is removed. After hydrolysis impurity solution composition shown in Table 2.
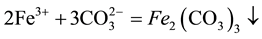 (1)
(1)
 (2)
(2)
3.3. Electrodeposition
Starting with the production of cobalt cathode pole piece, a titanium plate as an anode, the impurity resulting solution directly electrowinning current density of 240 A/m2, electrolyte temperature 55˚C - 60˚C, in return anolyte electrolysis and cleaning section the resulting electric cobalt surface roughness, the spectral analysis of its components, such as tables, Table 3, Figure 3 also lists the quality standards 1 and 1A # # electric cobalt electric cobalt [11] .
Table 3 shows full-immersion neutralized after hydrolysis direct electrowinning cobalt impurity, product quality can be made 1A electric cobalt standards. The electrolytic cobalt electricity obtained by the Formula (3)
Table 1. Content of each metal element in leaching solution.
Table 2. Content of each metal element in leaching solution after neutralization reaction to remove iron, aluminum.
Table 3. Cathode cobalt quality and criterion of GB6517-86.
obtain the current efficiency of the electrolysis process 90.01%.
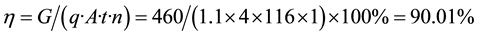 (3)
(3)
where: η―current efficiency, %; G―precipitation Electric Co Quality, g; q―cobalt electrochemical equivalent, 1.1 g Ah; A―current intensity, A; t―electrolysis cycle, h; n―cell number. Direct yields greater than 90% cobalt.
4. Conclusion
The use of sulfuric acid leaching-EW treatment of waste lithium 18605 lithium-ion battery technologies is simple. Cobalt leaching rate basically reached 100%, absolute yield of more than 90%. Leach solution after hydrolysis impurity can be directly electrowinning cobalt, simple process. We get 1A # electric cobalt comply GB6517- 86 by electrolyte. The current efficiency was 90.01%.
Cite this paper
LiangmouYu,BoShu,ShiwenYao, (2015) Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries. Advances in Chemical Engineering and Science,05,425-429. doi: 10.4236/aces.2015.54043
References
- 1. Debaraj, M., Kim, D.-J., Ralph, D.E., et al. (2008) Bioleaching of Metals from Spent Lithium Ion Secondary Batteries Using Acidithiobacillus ferrooxidans. Waste Management, 28, 333-338.
- 2. Jin, Y.-J., Mei, G.-J. and Li, S.-Y. (2006) Study on Cobaltous Recovery from Cathode Leachate of Lithium-Ion Battery by Salting Out. Acta Scientiae Circumstantiae, 26, 1122-1125. (In Chinese)
Wang, X.-F., Kong, X.-H. and Zhao, Z.-Y. (2001) Rcovery of Metal in Lithium ion Battery. Battery Bimonthly, 31, 14-15. (In Chinese) - 3. Nan, J.-M., Han, D.-M., Cui, M., et al. (2004) Recycling of Valuable Metal from Spent Li-Ion Batteries by Solvent Extraction. Battery Bimonthly, 34, 329-311. (In Chinese)
- 4. Lupi, C., Pasquali, M. and Dell’Era, A. (2005) Nickel and Cobalt Recycling from Lithium-Ion Batteries by Electrochemical Processes. Waste Management, 25, 215-220.
http://dx.doi.org/10.1016/j.wasman.2004.12.012 - 5. Wang, C.-Y., Qiu, D.-F., Chen, Y.-Q., et al. (2004) Status of Spent Batteries Recovery and Recycle. Nonferrous Metals, 5, 39-42. (in Chinese)
- 6. Xie, G.Y., Ling, Y. and Zhong, S. (2009) Overview of Recovery Techniques of Spent Lithium-Ion Batteries. Environmental Science & Technology, 32, 97-101.
- 7. Li, J.H., Shi, P.X., Wang, Z.F., et al. (2009) A Combined Recovery Process of Metals in Spent Lithium-Ion Batteries. Chemosphere, 77, 1132-1136.
http://dx.doi.org/10.1016/j.chemosphere.2009.08.040 - 8. Sun, L. and Qiu, K.Q. (2011) Vacuum Pyrolysis and Hydrometallurgical Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Journal of Hazardous Materials, 194, 378-384.
- 9. Li, L., Ge, J., Wu, F., et al. (2010) Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. Journal of Hazardous Materials, 176, 288-293.
- 10. Barbieri, E.M.S., Lima, E.P.C., Lelis, M.F.F., et al. (2014) Recycling of Cobalt from Spent Li-Ion Batteries as β- Co(OH)2 and the Application of Co3O4 as a Pseudocapacitor. Journal of Power Sources, 270, 158-165.
http://dx.doi.org/10.1016/j.jpowsour.2014.07.108 - 11. Zhao, J.M., Shen, X.Y., Deng, F.L., et al. (2011) Synergistic Extraction and Separation of Valuable Metals from Waste Cathodic Material of Lithium Ion Batteries Using Cyanex272 and PC-88A. Separation and Purification Technology, 78, 345-349.
http://dx.doi.org/10.1016/j.seppur.2010.12.024
上一篇:Solubility of Solids in Superc 下一篇:Solubility of Solids in Superc
最新文章NEWS
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Solubility of Solids in Supercritical Fluids: The Mendez-Santiago Teja Model Revisited
- Solventless Organic Reactive Crystallization at Mild Conditions
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Luminescent Characteristic of Organic Compound-Containing Inorganic Crystal at Room Temperature
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Tunable Polymorphic Transformation Temperature
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- A Convective Thin Layer Drying Model with Shrinkage for Kent Mango Slices
- Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Optimisation of Decolourisation Conditions of Crude Shea (Vitellaria paradoxa Gaertner F) Butter: Ye
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Solvent Extraction of Citric Acid with Different Organic Phases

