Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
Vol.05No.04(2015), Article ID:60196,10 pages
10.4236/aces.2015.54044
Gulu A. Bagirzade, Dilgam B. Tagiyev, Manaf R. Manafov
Institute of Catalysis and Inorganic Chemistry Named after M. Nagiyev, National Academy of Sciences of Azerbaijan, Baku, Azerbaijan
Email: mmanafov@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 June 2015; accepted 7 October 2015; published 12 October 2015
ABSTRACT
On the basis of kinetic data of 4-phenyl-o-xylene and 4-phenyl-o-tolunitrile ammoxidation, formation mechanism of the products was analyzed and generalized. It has been shown that dissociative adsorption of a substrate and mononitrile occurs on the centers with high heats of adsorption of oxygen and as a consequence, completely covered with it, competitive adsorption of NH3 and O2 occurs on the centers with low heats of adsorption of the latter; mononitrile and dinitrile are formed correspondingly from adsorbed fragments of both substrate and NH3, and tolunitrile and NH3; surface interaction of adsorbed fragments of substrate and O2 with low heat of adsorption gives imide and CO2; hydrolysis of dinitrile into imide occurs on centers completely covered with ammonia; imide decarboxylation occurs on the centers covered with it; oxidative destruction of tolunitrile occurs on centers covered with substrate and mononitrile.
Keywords:
Ammonolysis, 4-Phenyl-o-Xylene, Kinetics, Mechanism

1. Introduction
In papers [1] [2] kinetic regularities of vapor phase catalytic ammoxidation of both a substrate-4-phenyl-o-xy- lene and an intermediate 4-phenyl-o-tolunitrile were studied. A following scheme of conversions, constituting a total process of 4-phenyl-o-xylene ammoxidation on V-Sb-Bi-Zr/γ-Al2O3-oxide catalyst (Figure 1), was proposed in contributions [1] [3] [4] . The old analog wearing a patent character [5] exists in this field; however, the kinetic regularities of 4-phenyl-o-xylene ammoxidation on V2O5-CrO3/γ-Al2O3 catalyst have not been studied

Figure 1. The complete conversion of 4-phenyl-o-xylene under ammoxidation.
and a mechanism of products formation on the basis of kinetic data has not been discussed by authors. In paper [6] the main routes of catalytic oxidative conversions of some methyl derivatives of biphenyl in the gas phase, including 3,4-dimethylbiphenyl (4-phenyl-o-xylene) ammoxidation, were studied. In the patent [5] it is shown that for 10% of V2O5 - 5.6% CrO3 /g-Al2O3 catalyst at a temperature of 693K and a molar ratio of 4-phenyl-o-xy- lene: NH3:O2, equal to 1:39.71:6 turns out 4-phenylphthalonitrile with the 70% yield and conversion of the substrate in this case is 99%. Study of vapor oxidation and ammoxidation of 3,4-di-and 3,3', 4,4'-tetramethyldi- phenyls enables obtaining of the corresponding acid anhydrides, imides, nitriles of biphenylcarboxylic acids in a yield of 59 - 69 mol% [6] . The ammoxidation on many oxide catalysts is the exothermic catalytic reaction of o-xylene accompanied by the formation of major products, nitrites and imides. Therefore, an important object of research in the ammoxidation of xylene, and 4-brom- and 4-phenyl-o-xylene is the solution of the selectivity of the corresponding dinitrile and imide [7] . As a result of own study, it has been found that on the V-Sb-Bi-Zr/g- Al2O3-oxide catalyst, at high conversion of the starting 4-phenyl-o-xylene in single process [8] [9] , ammoxidation gives 80 mol% of 4-pfenylftalonitrile. Due to increased demands on the purity of the 4-pfenylftalonitrile while using and problem of the separation of hard separable impurities in the crystal 4-phenylftalimide, the variant of the technological design process with an average conversion and recycling unreacted 4-phenyl-o-xy- lene and intermediate 4-phenyl-o-tolunitrile is offered. In contrast to the single process, conducting of process with recirculation reduces quantity of the formed by-products, the share of deep oxidation and increases selectivity on a 4- phenyl phthalonitrile to 97.72% [8] .
Thus, the problem of the separation of hard separable crystal mixture of main products has been solved in the case of obtaining dinitrile from the substrate with the average conversion on the V-Sb-Bi-Zr/g-Al2O3-oxide catalyst, with the recirculation at higher concentrations of ammonia, and in the synthesis of imide with complete conversion of the substrate in a single process on V-Sb-Bi-Zr/g-Al2O3-oxide catalyst, using low concentrations of ammonia and water in the initial reaction mixture [9] [10] . As known, in general, and especially in cases where basic products are formed by competing pathways, to direct the reaction towards the important target substance, it is necessary to know its mechanism.
Major products of ammoxidation of aromatic compounds, having methyl groups at ortho-position, are both dinitrile and imide [10] - [12] , which are used in production of phthalocyanine pigments and dyes, photosensitizers of near IR spectrum for photodynamic cancer therapy [8] [9] , laser dyes, thermally stable polymers, medications, chemical means of plant protection and other fields of organic synthesis.
2. Methods and Apparatus
Kinetic measurements of 4-phenyl-o-xylene conversion and chromatographic separation of catalyzate components, and a quantitative calculation of their content were carried out in accordance with the earlier developed methods [1] . Kinetic measurements were performed in a setup with a vibrating fluidized-bed, gradientless flow reactor of 20-cm3 capacity made from 12Kh18N10T steel (Figure 2). Feed system of the reagents. Dosage of gaseous components of the initial reaction mixture, containing oxygen, ammonia and nitrogen (as gas-diluent), feed from cylinders, was being carried out by means of stopcocks {1} and a fine adjustment valve {5}. Gases cleaning and drying were being carried out in the gas columns {3}. Measurement of gases consumption was being performed by rheometers {4} with calibrated capillaries, and pressure in the system was being maintained constant by manostats {2}. 4-Phenyl-o-tolunitrile in liquid state by means of dispenser of syringe type {9} and gaseous reagents through the mixer {6} were being fed into the evaporator {7}, heated by oven {8}. The reaction assembly included a reactor {10} equipped with a thermowell, a grid (the same brand as the reactor), pre-

Figure 2. Scheme of gradientless installation with vibrating liquefied bed of catalyst.
venting entrainment of catalyst dust, as well as a vibrator {14} with the oscillation frequency of 50 Hz and an adjustable amplitude. Temperature in the reaction zone was being measured using a sliding thermocouple and recorded by electronic potentiometer KSP-4M. Temperature of block furnace {11} and the reactor were being controlled by a potentiometer KVP I-503 with accuracy to ±2˚. The furnace with a reactor was being placed in a thermostat {12}. Temperature in the thermostat was being maintained at 570 - 580 K by electrical spirals {13, 15} sufficient to prevent condensation of the reaction products. A system for collecting the reaction products included a heated switch {16}, with solvent trap of reaction products {17, 18}, the three-way cock {19}, a trap filled with sulfuric acid solution for quantitative recovery of unreacted ammonia {20}, a desiccant {21}, a gas sampler {22}, foam flow meter of flow rate {23}.The described scheme provides a material balance for all substances-reaction participants.
The products absorbed by 1,4-dioxane were determined on a Chrome-5 chromatograph equipped with an FID and a column of 1.2 m length. The column packing was Chromaton N-AW (0.2 - 0.25 mm) coated with a mixed stationary phase of Apiezon L (21%) and PEG 40000 (0.5%) or with Polisorb-1 (0.25 - 0.5 mm) alone. Carbon dioxide was determined on LKhM-8MD chromatograph with triethylene glycol butyrate supported on INZ-600 (calcined diatomaceous earth) as the stationary phase. Separation of O2 and N2 was carried out on the same chromatoraph using a parallel column packed with NaX.
3. Analysis of Experimental Products
In products of the reaction of 4-phenyl-o-xylene (I) ammoxidation on V-Sb-Bi-Zr/γ-Al2O3-oxide catalyst 4- phenyl-o-tolunitrile (II), 4-phenylphthalonitrile (III), 4-phenylpthalimide (IV), 4-phenylbenzonitrile (V), carbon dioxide, unreacted I, oxygen and the diluent gas-nitrogen were determined by gas chromatography. The reaction gases were successively passed through a 1,4-dioxane-filled trap for absorption of nitriles, and IV and sulfuric acid-filled trap for absorption of ammonia. The concentration of ammonia at the reactor outlet was determined by titration of unreacted sulfuric acid from the second trap. The carrier-gas (nitrogen) flow rate was 80 mL/min. The sample injection temperature was 353 K, and the temperature programming rate was 20 K/min.
4. Results and Discussion
In previous papers [2] [3] it has been shown, that at 4-phenyl-o-xylene ammoxidation on the oxide catalyst V-Sb-Bi-Zr/γ-Al2O3 due to the electronic factor of a phenyl group para-methyl groups are activated first, then meta-methyl groups are activated second with respect to substituent in an aromatic nucleus. By the way, the phenyl group is related to substituent of the third kind [13] , which may show both electron-donating and electron-withdrawing properties and, taking into account lack of a significant role of conjugation, i.e. electron-do- nating property of the phenyl group, due to the fact that resonance constants of phenyl and methyl groups, located at the para-position, are not significantly different [14] [15] , therefore should be noted that the phenyl group in 4-phenyl-o-xylene molecule reveals the electron-withdrawing character. As the phenyl group in 4- phenyl-o-xylene molecule does not affect on activation of meta-methyl group [16] , then on the basis of the electron-withdrawing property of phenyl group and a positive inductive (+J) effect of para-methyl group it can be said, that both influence on activation of para-methyl group in regard to phenyl substituent. Thus, as a result of activation first intermediate 4-phenyl-o-tolunitrile and then 4-phenylphthalonitrile are formed [1] [2] .
For unsubstituted o-xylene it does not matter which of two methyl groups is activated first in an aromatic nucleus. Therefore, as a result of activation of one from two methyl groups only o-tolunitrile is formed, which in its turn, due to activation of the second methyl group under the conditions of oxidative ammonolysis [17] [18] forms dinitrile. On the other hand, study of ammoxidation processes of 4-substituted o-xylene [1] [2] [7] [19] - [23] on the modified V-Sb-Bi/γ-Al2O3 catalysts, including V-Sb-Bi-Zr/γ-Al2O3-oxide contact, shows, that introduction of the phenyl group, or bromine atom into the aromatic nucleus of o-xylene negatively affects on its reactivity, where the phenyl substituent exerts the less negative influence [15] . In modern organic chemistry summative influence of substituents on the reaction center can be subdivided into induction and resonance (conjugation), i.e. the electronic and steric constituents, from which upon adsorption of 4-phenyl-o-xylene by the methyl group the steric factor [15] and upon activation (chemisorption) electronic factor [3] play a noticeable role.
During the primary activation of alkyl aromatic compounds with the metal oxide catalyst under the ammoxidation conditions lateral C-H bonds, being in α-position to an aromatic nucleus, are broken [24] . Activation mechanism under the action of primary center-of nucleophilic oxygen ion is shown in Figure 3.
As seen, efficiency of heterolytic dissociation of the C-H bond in the methyl group is ensured, first of all, by interaction of nucleophilic oxygen (О2−) with the protonated hydrogen. The heterolytic breakage of C-H bonds occurs also at activation of the second methyl group of 4-phenyl-o-xylene, since 4-phenilphthalonitrile is formed on V-Sb-Bi-Zr/γ-Al2O3-oxide catalyst mainly from 4-phenyl-o-tolunitrile. Thus, the primary interaction of 4- phenyl-o-xylene with the contact surface has an acid-base character, i.e. stage 1 (Figure 3) proceeds without changing an oxidation state of the metal Mn+ and is accompanied by separation of a proton from the substrate molecule (CH-acid) on nucleophilic catalyst center forming a surface compound of anionic type [3] [24] . For the oxidative reactions, mechanism of which comprises the acid-base stages, use of notions about the combined influence of redox and acid-base characteristics of the surface on the catalytic properties of substances is more productive [25] . Ammoxidation processes of toluene and its homologs, the primary interaction of which with the oxide catalyst can show the acid-base character [24] should be related to these reactions.
The purpose of the present article is to discuss a formation mechanism of the products of 4-phenyl-o-xylene ammoxidation, consistent with the observed kinetic regularities.
It is important to add, that as distinct from easiness of study of ammoxidation mechanism of olefinic and paraffinic hydrocarbons series [26] [27] , methodological difficulties, caused by comparatively high boiling and sublimation temperatures of initial substances and reaction products, highly detain conducting of detailed researches of ammoxidation mechanism of alkyl aromatic compounds [24] . The mechanism even for the simplest and sufficiently well-studied processes of ammoxidation remains still unexplained. An even greater difficulty is a study of the mechanism, as a result of which not only one but also two nitrile groups are formed, and imide group as well.
Earlier discussed by us, the formation mechanism of the products of o-xylene and p-xylene ammoxidation, consistent with the observed kinetic regularities, showed that in both cases dependence of the consumption rate of substrates allowed supposing their dissociative adsorption.
4-Phenyl-o-tolunitrile (II), 4-phenylphthalonitrile (III), 4-phenylphthalimide (IV), 4-phenylbenzonitrile (V) and CO2 are formed at vapor phase ammoxidation of 4-phenyl-o-xylene (I) in accordance with the Figure 1. Analysis of the experimental data showed, that formation and consumption rates of the basic components of the reaction of 4-phenyl-o-xylene ammoxidation on V-Sb-Bi-Zr/γ-Al2O3-oxide catalyst in a range of the partial pressures of oxygen and ammonia (whose measuring unit is kPa), exceeding their minimum values, are described by the Equations (1)-(7).

Figure 3. The mechanism of activation of the substrate with the catalyst under ammoxidation.
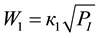 (1)
(1)
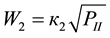 (2)
(2)
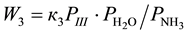 (3)
(3)
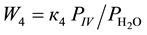 (4)
(4)
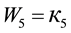 (5)
(5)
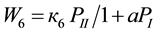 (6)
(6)
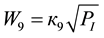 (7)
(7)
Here кi is a constant of the route rate (Figure 1).
Taking into account the observed dependence of the formation of the reaction products on the ratio of O2 and NH3 concentrations at values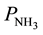 , lesser of some value, denoted as
, lesser of some value, denoted as 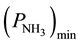 [1] , the rates of conversion of 4-phenyl-o-xylene into 4-phenyl-o-tolunitrile and 4-phenyl-o-tolunitrile into 4-phenylphthalonitrile are expressed by the Equations (8) and (9), respectively.
[1] , the rates of conversion of 4-phenyl-o-xylene into 4-phenyl-o-tolunitrile and 4-phenyl-o-tolunitrile into 4-phenylphthalonitrile are expressed by the Equations (8) and (9), respectively.
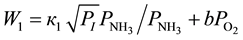 (8)
(8)
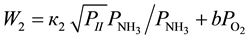 (9)
(9)
In the indicated range of ammonia concentrations the routes 7 and 8 are added according to the Figure 1, the rates of which are described by the Equations (10) and (11).
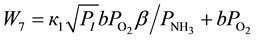 (10)
(10)
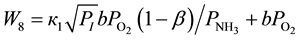 (11)
(11)
Here b is a share of 4-phenyl-o-xylene, which at small concentrations of ammonia is converted to 4-phe- nylphthalimide; b is a constant reflecting ratio of the adsorption equilibrium constants of O2 and NH3.
 (12)
(12)
In accordance with Figure 1, Wsum is independent on NH3 concentration and obeys the half-order equation on 4-phenyl-o-xylene. Such dependence allows assuming a dissociative adsorption of 4-phenyl-o-xylene at centers, which at  is completely covered with oxygen. At the same time the adsorbed 4-phenyl-o-xylene fragment with probability к9/к1 + к9 along the route 9 is oxidized to CO2 and H2O, with probability к1/к1 + к9 leads to the formation of 4-phenyl-o-tolunitrile, and at small values of
is completely covered with oxygen. At the same time the adsorbed 4-phenyl-o-xylene fragment with probability к9/к1 + к9 along the route 9 is oxidized to CO2 and H2O, with probability к1/к1 + к9 leads to the formation of 4-phenyl-o-tolunitrile, and at small values of  also produces 4-phenylphthalimide, CO2 and H2O. Correlation of the formation rates of 4-phenyl-o-tolunitrile on the route 1 (W1) and other products of the reaction on the routes 7 and 8 (W7 + W8) depends on the ratio of
also produces 4-phenylphthalimide, CO2 and H2O. Correlation of the formation rates of 4-phenyl-o-tolunitrile on the route 1 (W1) and other products of the reaction on the routes 7 and 8 (W7 + W8) depends on the ratio of . This together with independence of the conversion rate of 4-phenyl-o-xylene on
. This together with independence of the conversion rate of 4-phenyl-o-xylene on  gives a reason to suggest, that NH3 is adsorbed on other centers, than 4-phenyl-o-xylene, besides in this adsorption it competes with oxygen, completely displacing it at the high values of
gives a reason to suggest, that NH3 is adsorbed on other centers, than 4-phenyl-o-xylene, besides in this adsorption it competes with oxygen, completely displacing it at the high values of . Obviously, these centers (Z) have less adsorption heat of oxygen than the adsorption centers of 4-phenyl-o-xylene (Y).
. Obviously, these centers (Z) have less adsorption heat of oxygen than the adsorption centers of 4-phenyl-o-xylene (Y).
Taking into consideration the foregoing, sequence of the steps leading to adsorption of 4-phenyl-o-xylene and its complete oxidation on the route 9 can be represented in the following way:
1) 
2) 
3) 
4¢) 
5) 
6¢) 
Here, step 1) is the equilibrium stage, the stages with dashes are instant [28] , all other stages are slow, and C6H5 = Ph.
Equilibriumly adsorbed on centers ZO2 and NH3 completely cover their.
7) 
8) 
Hence:
 (13)
(13)
Then O2 and NH3 covering can be calculated as follows.
 (14)
(14)
 (15)
(15)
Here, Ki is an equilibrium constant of the reaction stage.
If YO2CHPhC6H3CH3 interacts with ZNH3, then 4-phenyl-o-tolunitrile is formed, in the case of interaction with ZO2 either 4-phenylphthalimide or CO2 and H2O are formed on the routes 7 and 8 (Figure 1).
9¢) 
10¢) 
11¢) 
12¢) 
13¢) 
14¢) 
Covering of Y centers by the fragments of organic origin is little.
If consumption rate of YOCHPhC6H3CH3 in the stages 2) and 3) is approximately equals to consumption rate of YOH2 in the stage 5), then their concentrations are also equal with each other, i.e. [YOCHPhC6H3CH3] » [YOH2].
Then, from the equilibrium stage 1) and condition of full covering of Y centers by oxygen ([YO] ≈ 1) we obtain the expressions (16) and (17).
 (16)
(16)
 (17)
(17)
The consumption rate of 4-phenyl-o-xylene on the route 9, taking into account defining of this direction slow stage 3) at [YO] ≈ 1, is determined by the Equation (18), and on the routes 1, 7 and 8, taking into account defining of the direction slow stage 2) at [YO] ≈ 1 by the Equation (19).
 (18)
(18)
 (19)
(19)
 , (20)
, (20)
Here, ki is a rate constant of the reaction stage.
The values W1 and [W7 + W8], taking into account coverings of NH3 and O2, which determine the routes 1 and [7 + 8] are found by the Equations (21) and (22).
 (21)
(21)
 (22)
(22)
 (23)
(23)
In turn, the rates ratio of the routes 7 and 8 is defined by relation of the constants of the stages 11¢) and 13¢).
 (24)
(24)
 (25)
(25)
On the Y centers, analogously to adsorption of 4-phenyl-o-xylene, 4-phenyl-o-tolunitrile is dissociatively adsorbed.
15) 
16) 
17¢) 
Since the formation rates of YOCHPhC6H3CN and YOH2 at the stage 15) are the same, then [YOCHPhC6H3CN] = [YOH2].
Therefore, from the equilibrium of the stage 15) and the conditions of full coverage of the Y centers by oxygen ([YO] ≈ 1) follows
 (26)
(26)
hence
 (27)
(27)
Then, the consumption rate of 4-phenyl-o-tolunitrile on the route 2, taking into account defining of this direction slow stage 16) at [YO] ≈ 1, is determined by the Equation (28).
 (28)
(28)
Taking into consideration NH3 covering, which determines the route 2, the reaction rate is determined by the Equation (29).
 (29)
(29)
 (30)
(30)
A character of kinetic dependencies of hydrolysis of 4-phenylphthalonitrile and reverse reaction of 4-phe- nylphthalimide ammonolysis on water and ammonia concentrations shows, that these reactions occur on the catalytic centers of different nature. Ammonia on them competes with water, and ammonia covering is much more than covering by adsorbed water and close to 1.
18) 
19) 
20) 
21) 
Here, the stages 18), 19) and 21) are equilibrium stages; stage 20) is reversible, but not the equilibrium.
Covering with adsorbed water is calculated by the Equation (31).
 (31)
(31)
Then, the reaction rate on the route 3 in the forward direction, taking into account defining of the direction stage 20), is determined by the Equation (32).
 (32)
(32)
 (33)
(33)
The equilibrium constant of the stage 21), taking into account [X(NH3)] » 1 is equal to:
 (34)
(34)
Hence, covering with the intermediate compound:
 (35)
(35)
Then, the reaction rate on the route 4 of the reverse direction of 4-phenylphthalimide ammonolysis, taking into account defining stage 20), is equal to:
 (36)
(36)
Consequently,
 (37)
(37)
At equilibrium,
 (38)
(38)
Then,
 (39)
(39)
and equilibrium constant of the hydrolysis reaction of 4-phenylphthalonitrile may be expressed in the following way (40).
 (40)
(40)
If to judge on the observed kinetic regularities [1] [2] , then formation of 4-phenylbenzonitrile both from 4- phenylphthalimide and from 4-phenyl-o-tolunitrile occurs on other centers than ammoxidation. Existence of such centers on the surface of the multicomponent catalyst is quite possible. Therefore, a character of the kinetic dependences of 4-phenylphthalimide decarboxylation shows, that this reaction takes place on of catalytic centers (Ω), covered with imide.
22) 
23) 
24¢) 
The consumption rate of 4-phenylphthalimide on the route 5, taking into account defining of the direction slow stage 23) at covering with imide, closed to 1,
 (41)
(41)
Consequently,
 (42)
(42)
Formation of 4-phenylbenzonitrile at the oxidative destruction (oxidative demethylation) of 4-phenyl-o-tolu- nitrile is impeded by 4-phenyl-o-xylene, and therefore, 4-phenyl-o-xylene and 4-phenyl-o-tolunitrile equilibriumly adsorbing on Λ centers cover them.
25) 
26) 
Then coverings of 4-phenyl-o-xylene and 4-phenyl-o-tolunitrile are determined by the equations (43, 44).
 (43)
(43)
 (44)
(44)
Sequence of the stages of 4-phenyl-o-tolunitrile oxidative destruction on the route 6 can be represented as follows.
27) 
28) 
29¢) 
30¢) 
31¢) 
32) 
From the foregoing follows, that
 (45)
(45)
Taking into consideration this, and because of the relatively low yields of 4-phenylbenzonitrile even on the routes 5 and 6 (Figure 1) may assume, that Λ centers covering with the fragments of inorganic origin are very small (46).
 (46)
(46)
From the stage 26) for [Λ(CH3PhC6H3CN)], taking into account sum of the centers numbers, equal to unity, we obtain the following expressions (47) and (48).
 (47)
(47)
 (48)
(48)
Then, the consumption rate of Λ(CH3PhC6H3CN)-adsorbed 4-phenyl-o-tolunitrile at PhC6H4CN formation on the slow stage 28) is determined by the equation (49).
 (49)
(49)
Furthermore,
 (50)
(50)
Hence,
 (51)
(51)
 (52)
(52)
Here a is a constant reflecting ratio of the constants of adsorption equilibrium of 4-phenyl-o-xylene and 4- phenyl-o-tolunitrile.
5. Conclusions
1) Ensuing from the proposed stage schemes of 4-phenyl-o-xylene ammoxidation mechanism, kinetic equations are in qualitative agreement with experimental data.
2) In deriving of the equations, physical meaning of a number of constants appearing in them is revealed.
3) The mechanism of formation of the main products as in the competing ways and its mutual transformation in each other are discussed.
4) The mechanism shows that the adsorbed 4-phenyl-o-xylol fragment as the general activated complex in interaction with ZNH3 forms 4-phenyl-o-tolunitrile, which in its turn transforms in 4-pfenylftalonitrile and in the case of interaction with ZO2 forms 4-pfenylftalimide.
5) Conducted research provides an opportunity to create an effective single technological process of obtaining 4-pfenylftalonitrile, by the ammoxidation reaction of 4-phenyl-o-xylene.
Acknowledgements
Used in this work software package OptimMe was developed by the support of Science Foundation of “SOCAR” under the grant project ET-27 (15/10/2014) at the Institute of Catalysis and Inorganic Chemistry named after Acad. M. F. Nagiyev.
Cite this paper
Gulu A.Bagirzade,Dilgam B.Tagiyev,Manaf R.Manafov, (2015) Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene. Advances in Chemical Engineering and Science,05,430-440. doi: 10.4236/aces.2015.54044
References
- 1. Bagirzade, G.A. (2012) Preparation of 4-Phenylphthalonitrile by Vapor-Phase Ammoxidation of 4-Phenyl-o-Xylene: Reaction Kinetics. Petroleum Chemistry, 52, 105-112.
http://dx.doi.org/10.1134/S0965544112020028 - 2. Bagirzade, G.A., Tagiyev, D.B. and Manafov, M.R. (2014) Synthesis of 4-Phenyl-phthalonitrile by Vapor-Phase Catalytic Ammoxidation of Intermediate 4-Phenyl-o-Tolunitrile: Reaction Kinetics. Modern Research in Catalysis, 3, 6-11.
http://dx.doi.org/10.4236/mrc.2014.31002 - 3. Bagirzade, G.A. (2012) On Sequence of Activation of Methyl Groups of 4-Phenyl-and 4-Brom-o-Xylenes at the Heterogeneous Catalytic Oxidative Ammonolysis. Izvestia Vysshykh Uchebnykh Zavedeniy Khimia I Khimicheskay Tekhnologia, 55, 23-27.
- 4. Bagirzade, G.A., Tagiyev, D.B., Sheinin, V.E. and Magerramova, Z.Y. (2013) Kinetic Model of Oxidative Ammonolysis of 4-Phenyl-o-Xylene. Azerbaijan Chemical Journal, 3, 47-54.
- 5. Pat 632702 Belg (1963) Production of Aromatic Nitriles. Chemical Abstracts, 61, 3032 h.
- 6. Shapovalov, A.A., Koshel, S.G., Sembaev, D.Kh., Postanova, M.V. and Koshel, G.N. (1999) Vapor-Phase Oxidation and Ammoxidation of Some Methyl Derivatives of Biphenyl. Russian Journal of Applied Chemistry, 72, 1315-1319.
- 7. Bagirzade, G.A., Tagiyev, D.B. and Fatullayeva, S.S. (2014) Transformation Pathways of o-Xylene and Its 4-Substituted Derivatives in the Course of Vapor-Phase Oxidative Ammonolysis. Russian Journal of Applied Chemistry, 87, 1674-1679.
http://dx.doi.org/10.1134/S1070427214110172 - 8. Bagirzade, G.A. (2011) Resource-Saving and Power-Efficient Technologies in Chemical and Petrochemical Industries. Abstracts of Papers of III International Conference of the Mendeleev Russian Chemical Society, D. Mendeleev University of Chemical Technology of Russia, Moscow, 133-135. (In Russian)
- 9. Bagirzade, G.A. and Tagiyev, D.B. (2012) Chemical Technology and Biotechnology of New Materials and Products. Abstracts of Papers of IV International Conference of the Mendeleev Russian Chemical Society, D. Mendeleev University of Chemical Technology of Russia, Moscow, 9-11. (In Russian)
- 10. Bagirzade, G.A., Sheinin, V.E. and Magerramova, Z.Y. (2009) Resource-and Energy-Saving Technology in the Chemical and Petrochemical Industry. Abstracts of Papers of I International Conference of the Mendeleev Russian Chemical Society, D. Mendeleev University of Chemical Technology of Russia, Moscow, 94-95. (In Russian)
- 11. Bagirzade, G.A. (2010) Innovative Chemical Technologies and Biotechnologies of Materials and Products. Abstracts of Papers of II International Conference of the Mendeleev Russian Chemical Society, D. Mendeleev University of Chemical Technology of Russia, Moscow, 197-198. (In Russian)
- 12. Bagirzade, G.A. and Tagiyev, D.B. (2013) Resources-and Energy-Saving Technologies in the Chemical and Petrochemical Industry. Abstracts of Papers of V International Conference of the Mendeleev Russian Chemical Society, D. Mendeleev University of Chemical Technology of Russia, Moscow, 56-58. (In Russian)
- 13. Plemenkov, V.V. (1997) Electronic and Steric Structure of Monofunctional Cyclo-Propanes. Russian Journal of Organic Chemistry, 33, 849-859.
- 14. Palm, V.A. (1974) Introduction to Theoretical Organic Chemistry. Higher School, Moscow. (In Russian)
- 15. Bagirzade, G.A. (2009) On the Reactivity of o-Xylene and It 4-Substituted at the Heterogeneous Catalytic Oxidative Ammonolysis. Izvestia Vysshykh Uchebnykh Zavedeniy Khimia I Khimicheskay Tekhnologia, 52, 47-49.
- 16. Ravdel, A.A. and Ponomarevf, A.M. (1983) Short Handbook of Chemical-Physical Parameters. Khimiya, Leningrad. (In Russian)
- 17. Rizayev, R.G., Scheinin, V.E., Allahkulu, A.O. and Avetisov, A.K. (1985) Kinetics of Oxidative Ammonolysis of o-Xylene I. Transformations of o-Xylene and o-Tolunitrile. Kinetics and Catalysis, 26, 345-348.
- 18. Rizayev, R.G., Scheinin, V.E., Allahkulu, A.O. and Avetisov, A.K. (1986) Kinetics of Oxidative Ammonolysis of o-Xylene II. Formation of By-Products. Kinetics and Catalysis, 27, 339-345.
- 19. Bagirzade, G.A. (2010) Kinetics of Oxidative Ammonolysis of 4-Brom-o-Xylene I. Transformations of 4-Brom-o-Xylene and 4-Brom-o-Tolunitrile. Russian Journal of General Chemistry, 80, 1672-1676.
http://dx.doi.org/10.1134/S1070363210080177 - 20. Bagirzade, G.A. (2010) Kinetics of Oxidative Ammonolysis of 4-Brom-o-Xylene II. Formation of By-Products. Russian Journal of General Chemistry, 80, 1779-1785.
http://dx.doi.org/10.1134/S1070363210090100 - 21. Bagirzade, G.A. (2013) Kinetics of Oxidative Ammonolysis of 4-Brom-o-Xylene III. Transformation of 4-Brom-o-Tolunitrile as Substrate. Russian Journal of General Chemistry, 83, 492-495.
http://dx.doi.org/10.1134/S1070363213030158 - 22. Bagirzade, G.A. (2014) Kinetics of Oxidative Ammonolysis of 4-Brom-o-Xylene IV. Mechanism of Formation of the Reaction Products. Russian Journal of General Chemistry, 84, 1079-1084.
http://dx.doi.org/10.1134/S1070363214060048 - 23. Bagirzade, G.A. and Tagiyev, D.B. (2014) Kinetics of Oxidative Ammonolysis of 4-Brom-o-Xylene V. Syntesis of 4-Bromphtalonitrile. Russian Journal of General Chemistry, 84, 1085-1090.
- 24. Rizayev, R.G., Mamedov, E.A., Scheinin, V.E. and Vislovsky, V.P. (1992) Heterogeneous Catalysis in the Production of Aromatic Nitriles. Elm, Baku. (In Russian)
- 25. Golodets, G.I. (1985) Some Problems in the Theory of Heterogeneous Catalytic Partial Oxidation. Problems of Kinetics and Catalysis, 19, 28-58.
- 26. Sokolovsky, V.D. (1985) Mechanism of Selective Ammoxidation of Lower Paraffins. Problems of Kinetics and Catalysis, 19, 99-119.
- 27. Sadovsky, A.S. and Gelbshteyn, A.I. (1985) Propylene Ammoxidation (Mechanism and Kinetics Questions). Problems of Kinetics and Catalysis, 19, 119-131.
- 28. Kiperman, S.L. (1979) Fundamentals of Chemical Kinetics in Heterogeneous Catalysis. Khimiya, Moscow. (In Russian)
上一篇:Solubility of Solids in Superc 下一篇:Recycling of Cobalt by Liquid
最新文章NEWS
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Solubility of Solids in Supercritical Fluids: The Mendez-Santiago Teja Model Revisited
- Solventless Organic Reactive Crystallization at Mild Conditions
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Luminescent Characteristic of Organic Compound-Containing Inorganic Crystal at Room Temperature
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Tunable Polymorphic Transformation Temperature
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- A Convective Thin Layer Drying Model with Shrinkage for Kent Mango Slices
- Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Optimisation of Decolourisation Conditions of Crude Shea (Vitellaria paradoxa Gaertner F) Butter: Ye
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Solvent Extraction of Citric Acid with Different Organic Phases
