Using Row Reduced Echelon Form in Balancing Chemical Equations
Vol.06No.04(2016), Article ID:72775,12 pages
10.4236/alamt.2016.64014
R. O. Akinola1*, S. Y. Kutchin1, I. A. Nyam1, O. Adeyanju2
1Department of Mathematics, Faculty of Natural Sciences, University of Jos, Jos, Nigeria
2Department of Chemistry, Faculty of Natural Sciences, University of Jos, Jos, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 5, 2016; Accepted: December 12, 2016; Published: December 15, 2016
ABSTRACT
In an earlier paper published in the Journal of Natural Sciences Research in 2015 on how to balance chemical equations using matrix algebra, Gabriel and Onwuka showed how to reduce the resulting matrix to echelon form using elementary row operations. However, they did not show how elementary row operations can be used in reducing the resulting echelon matrix to row reduced echelon form. We show that the solution obtained is actually the nullspace of the matrix. Hence, the solution can be infinitely many. In addition, we show that instead of manually using row operations to reduce the matrix to row reduced echelon form, software environments like octave or Matlab can be used to reduce the matrix directly. In all the examples presented in this paper, we reduced all matrices to row reduced echelon form showing all row operations, which was not clearly stated in the Gabriel and Onwuka paper. Most importantly, with the availability of Mathematical software, we show that we do not need to carry out these row operations by brute force.
Keywords:
Nullspace, Chemical Equations

1. Introduction
According to Risteski [1] , a chemical reaction is an expression showing a symbolic re- presentation of the reactants and products that is usually positioned on the left and right hand sides of a particular chemical reaction. Substances that takes part in a che- mical reaction are represented by their molecular formula and their symbolic repre- sentation is also regarded as a chemical reaction [2] . A chemical reaction can either be reversible or irreversible. These differs from Mathematical equations in the sense that while a single arrow (in the case of an irreversible reaction) or a double arrow points in the forward and backward directions of both the reactants and products (in the case of a reversible reaction) connects chemical reactions [3] , an equality sign links the left and right hand sides of a Mathematical equation. “The quantitative and qualitative know- ledge of the chemical processes which estimates the amount of reactants, predicting the nature and amount of products and determining conditions under which a reaction takes place is important in balancing a chemical reaction. Balancing Chemical reaction is an excellent demonstative and instructive example of the inter-connectedness be- tween Linear Algebra and Stoichiometric principles” [4] .
If the number of atoms of each type of element on the left is the same as the number of atoms of the corresponding type on the right, then the chemical equation is said to be balanced [3] , otherwise it is not. The qualitative study of the relationship between reactants in a chemical reaction is termed Stoichiometry [5] . Tuckerman [6] mentioned two methods for balancing a Chemical reaction: by inspection and algebraic. The ba- lancing-by-inspection method involves making successive intelligent guesses at making the coefficients that will balance an equation equal and continuing until the equation is balanced [4] . For simple equations, this procedure is straight forward. However, according to [7] , there is need for a “step-by-step” approach which is easily applicable and can be mastered; rather than the haphazard hoping of inspection or a highly refined inspection. In addition, balancing-by-inspection method makes one to believe that there is only one possible solution rather than an infinite number of solutions which the method proposed in this paper illustrates. The algebraic approach circumvents the above loo- pholes provided in the inspection method and can handle complex chemical reac- tions.
The algebraic approach discussed in [6] , involves putting unknown coefficients in front of each molecular species in the equation and solving for the unknowns. This is then followed by writing down the balance conditions on each element. After which he lets one of the unknowns to be one and takes turns to obtain the coefficients of the remaining unknowns. In the proposed approach, instead of setting one of the unknowns to zero, we write out the set of equations in matrix form, obtain a homogeneous system of equations. Since the system of equations is homogeneous, the solution obtained is in the nullspace of the corresponding matrix. We then perform elementary row operations on the matrix to reduce it to row reduced echelon form. We also show the use of software environments like Matlab/octave to reduce the corresponding matrix to row reduced echelon form using the rref command. This approach surpasses those in [4] ; in the sense that we do not need to manually reduce the matrix to echelon form as shown in that paper. In that paper, they showed how the corresponding matrix is reduced to echelon form but did not use elementary row operations to convert it to row reduced echelon form.
In the next section, we state two well known results partaining echelon form and row reduced echelon form.
2. Methodolology
In this section, we state well known results about echelon form and row reduced echelon form. We will not bother about the algorithm as this is readily available in most Linear Algebra textbooks.
Lemma 2.1.: The number of nonzero rows and columns are the same in any echelon form produced from a given matrix 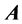 by elementary row operations, irrespective of the sequence of row operations used.
by elementary row operations, irrespective of the sequence of row operations used.
Given an 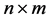 matrix
matrix ,
,
1. Use Gauss elimination to produce an echelon form from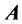 .
.
2. Use the bottom-most non zero entry  in each leading column of the echelon form, starting with the rightmost leading column and working to the left, so as to eliminate all non-zero entries in that column strictly above that entry one.
in each leading column of the echelon form, starting with the rightmost leading column and working to the left, so as to eliminate all non-zero entries in that column strictly above that entry one.
Definition 2.1 An  matrix
matrix 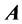 is said to be in row reduced echelon from when:
is said to be in row reduced echelon from when:
1. It is in echelon form (with  non-zero rows, say)
non-zero rows, say)
2. The 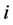 th leading column equals
th leading column equals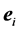 , the
, the 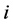 th column of the identity matrix of order
th column of the identity matrix of order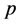 , for
, for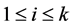 .
.
The next result which can be found in [8] , describes the uniqueness of the row reduced echelon form. It is the uniqueness of the row reduced echelon form that makes it a tool for finding the nullspace of a matrix.
Theorem 2.1 (Row Reduced Echelon Form): Each matrix has precisely one row reduced echelon form to which it can be reduced by elementary row operations, regardless of the actual sequence of operations used to produce it.
Proof. See [8] .
3. Worked Examples
Example 3.1.: Rust is formed when there is a chemical reaction between iron and oxygen. The compound that is formed is a reddish-brown scales that cover the iron object. Rust is an iron oxide whose chemical formula is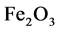 , so the chemical for- mula for rust is
, so the chemical for- mula for rust is
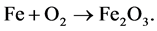
Balance the equation.
In balancing the equation, let 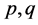 and
and  be the unknown variables such that
be the unknown variables such that
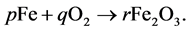
We compare the number of Iron (Fe) and Oxygen (O) atoms of the reactants with the number of atoms of the product. We obtain the following set of equations:


The homogeneous system of equations becomes

From the above, the matrix  is already in the echelon form
is already in the echelon form , with two pivots 1 and 2 but not in row reduced echelon form, even though there is a zero above the second pivot 2. However, to reduce it to row reduced echelon form
, with two pivots 1 and 2 but not in row reduced echelon form, even though there is a zero above the second pivot 2. However, to reduce it to row reduced echelon form ; all the pivots
; all the pivots
must be one. Hence, we replace row two with half row two, that is  to yield,
to yield,
 (1)
(1)
Thus,  becomes
becomes

Upon expanding, we have


the nullspace solution

There are three pivot variables  and one free variable
and one free variable . If we choose
. If we choose , then
, then . To avoid fractions, we can also let
. To avoid fractions, we can also let , so that
, so that  and
and
 . We remark that these are not the only solutions since there is a free variable
. We remark that these are not the only solutions since there is a free variable , the nullspace solution is infinitely many. Therefore, the chemical equation can be balanced as
, the nullspace solution is infinitely many. Therefore, the chemical equation can be balanced as

or

Example 3.2.: Ethane  burns in oxygen to produce carbon (IV) oxide
burns in oxygen to produce carbon (IV) oxide  and steam. The steam condenses to form droplets of water viz;
and steam. The steam condenses to form droplets of water viz;

balance the equation.
Let the unknowns be  and
and , such that
, such that

We compare the number of Carbon (C), Hydrogen (H) and Oxygen (O) atoms of the reactants with the number of atoms of the products. We obtain the following set of equations:



In homogeneous form,

In the first step of elimination, replace row two by row two minus three times row one, i.e.,  to yield,
to yield,

Exchange row two with row three or vice versa to reduce  to echelon form
to echelon form ,
,

In the next set of operations that we will carry out to reduce  to
to , we perform row operations that will change the entries above the pivots to zero; Replace row one by three times row two plus two times row three i.e.,
, we perform row operations that will change the entries above the pivots to zero; Replace row one by three times row two plus two times row three i.e.,  and replace row one with three times row one plus row three
and replace row one with three times row one plus row three  to yield
to yield

The last operation that will give us , is to reduce all the pivots to unity, that is replace row one with one-sixth row one, row two with one-sixth row two and row three with one-third row three to obtain
, is to reduce all the pivots to unity, that is replace row one with one-sixth row one, row two with one-sixth row two and row three with one-third row three to obtain
 (2)
(2)
The solution to  reduces to
reduces to  where
where  is actually the nullspace of
is actually the nullspace of  which is equivalent to the nullspace of
which is equivalent to the nullspace of . Hence,
. Hence,

Upon expanding, we have



the nullspace solution

There are three pivot variables  and one free variable
and one free variable . Let
. Let , so that
, so that  and
and . We remark that this is not the only solution since there is a
. We remark that this is not the only solution since there is a
free variable , the nullspace solution is infinitely many. Therefore, the chemical equation can be balanced as
, the nullspace solution is infinitely many. Therefore, the chemical equation can be balanced as

Example 3.3.: Sodium hydroxide (NaOH) reacts with sulphuric acid  to yield sodium sulphate
to yield sodium sulphate  and water,
and water,

Balance the equation.
In balancing the equation, let  and
and  be the unknown variables such that
be the unknown variables such that

We compare the number of Sodium (Na), Oxygen (O), Hydrogen (H) and Sulphur (S) atoms of the reactants with the number of atoms of the products. We obtain the following set of equations:




Re-writing these equations in standard form, we have a homogeneous system  of linear equations with
of linear equations with  and
and 




or

The augmented system becomes

Since the right hand side is the zero vector, we work with the matrix  because any row operation will not change the zeros.
because any row operation will not change the zeros.
Replace row 2 with row two minus row one i.e., . Similarly, replace row three with row three minus row one i.e.,
. Similarly, replace row three with row three minus row one i.e., . These first set of row operations reduces
. These first set of row operations reduces  to
to

In the second set of row operations, we replace row three by two times row three minus row two or  and replace row four by four times row four minus row two or
and replace row four by four times row four minus row two or  to yield
to yield

In the third stage of the elimination process, we replace row four with 3 times row four plus row three i.e.,  to yield the row echelon matrix or upper triangular
to yield the row echelon matrix or upper triangular ,
,

We now reduce  to row reduced echelon form
to row reduced echelon form  as follows: First, we reduce the pivots to unity in rows two and three via
as follows: First, we reduce the pivots to unity in rows two and three via  and
and  to obtain
to obtain

Replace row one by row one plus two times row three i.e.,  and row two by row two plus half row three, that is
and row two by row two plus half row three, that is . These two operations
. These two operations
replaces all nonzeros above the pivots to zero resulting in the row reduced echelon form 
 (3)
(3)
The solution to  reduces to
reduces to  where
where  is actually the nullspace of
is actually the nullspace of  which is equivalent to the nullspace of
which is equivalent to the nullspace of . Hence,
. Hence,

Upon expanding, we have



the nullspace solution

There are three pivot variables  and one free variable
and one free variable . We set
. We set , so that
, so that  and
and . We remark that this is not the only solution since there is a free variable
. We remark that this is not the only solution since there is a free variable , the nullspace solution is infinitely many. Therefore, the chemical equation can be '”balanced” as
, the nullspace solution is infinitely many. Therefore, the chemical equation can be '”balanced” as

Example 3.4.: Using row reduced echelon form, balance the following chemical reaction:

Let  and
and  be the unknown variables such that
be the unknown variables such that

We obtain the following set of equations for each of the elements:




The corresponding matrix becomes

The following row operations ,
,  and
and  reduces
reduces  to
to

In the same vein, the following row operations  and
and  reduces the above matrix to
reduces the above matrix to

Finally,  reduces the matrix to echelon form
reduces the matrix to echelon form

There are three pivots respectively . Hence, to reduce the matrix to row reduced echelon form, we make sure the entries above the pivots are zero and then change the pivots to unity. The row operations
. Hence, to reduce the matrix to row reduced echelon form, we make sure the entries above the pivots are zero and then change the pivots to unity. The row operations ,
,  and
and
 changes the nonzero entries above the pivots to zero so that
changes the nonzero entries above the pivots to zero so that  reduces to
reduces to

The row operations ,
,  and
and  leads to the row reduced echelon form
leads to the row reduced echelon form
 (4)
(4)
Therefore, the solution  to
to  becomes
becomes

For simplicity, we equate  to one so that
to one so that . This actually shows that the equation was balanced in the first place.
. This actually shows that the equation was balanced in the first place.
4. Using Matlab or Octave rref Command
In this section, we use octave to reduce each of the matrices considered in the last section to row reduced echelon form. We remark that just as predicted by the theory, row exchanges does not change the outcome of row reduced echelon form. This means that if you interchange any of the row of each of the matrices in the four examples, the rref will be the same.
Example 4.1.: Type the matrix  and
and . This gives the same
. This gives the same  as in (1) as
as in (1) as

Example 4.2.:  and
and . This gives the same
. This gives the same  as in (2) as
as in (2) as

Example 4.3.:  and
and  . This gives the same
. This gives the same  as in (3) as
as in (3) as

Example 4.4.:  and
and  . This gives the same
. This gives the same  as in (4) as
as in (4) as

In the next example, we illustrate the power of the rref command.
Example 4.5.: Consider balancing the following chemical reaction from [6]

Let the unknown coefficients be  such that
such that

We write down the balance conditions on each element as
Sodium: .
.
Chlorine: .
.
Sulphur: .
.
Oxygen: .
.
Hydrogen: 
After transposing, the above system of equations can be written in the form  as
as

Using Matlab or Octave  command,
command,  reduces to
reduces to  as
as

If we set , then
, then  and
and . The balanced equation be- comes
. The balanced equation be- comes

5. Conclusion
In this paper, we have shown how to balance chemical equations using row reduced echelon form. In actual fact, the echelon form alone could have been used and we still have the same solution but reducing it to rref makes the solution easily deduced. This paper improves on the work of Gabriel and Onwuka and we show that the octave/ Matlab rref command can be used to confirm the correctness of the final output on the one hand or as a stand alone.
Cite this paper
Akinola, R.O., Kut- chin, S.Y., Nyam, I.A. and Adeyanju, O. (2016) Using Row Reduced Echelon Form in Balancing Chemical Equations. Advances in Linear Algebra & Matrix Theory, 6, 146- 157. http://dx.doi.org/10.4236/alamt.2016.64014
References
- 1. Risteski, I.B. (2009) A New Singular Matrix Method for Balancing Chemical Equations and Their Stability. Journal of the Chinese Chemical Society, 56, 65-79.
https://doi.org/10.1002/jccs.200900011 - 2. Roa, C.N.R. (2007) University General Chemistry: An Introduction to Chemistry Science. Rajiv Beri for Macmillian India Ltd., 17-41.
- 3. Lay, D.C. (2006) Linear Algebra and Its Applications. 17-120.
- 4. Gabriel, C.I. and Onwuka, G.I. (2015) Balancing of Chemical Equations Using Matrix Algebra. Journal of Natural Sciences Research, 3, 29-36.
- 5. Hill, J.W., Mecreary, T.W. and Kolb, D.K. (2000) Chemistry for Changing Times. Pearson Education Inc., 123-143.
- 6. Tuckerman, M.E. (2011).
http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_2/node3.html - 7. Hutchings, L., Peterson, L. and Almasude, A. (2007) The Journal of Mathematics and Science: Collaborative Explorations, 9, 119-133.
- 8. Noble, B. and Daniel, J.W. (1988) Applied Linear Algebra. 3rd Edition, 90-97, 103, 140-149.
上一篇:Jordan Γ*-Derivation on 下一篇:Tight Monomials with t-Value
最新文章NEWS
- On Characterization of Poised Nodes for a Space of Bivariate Functions
- Least-Squares Solutions of Generalized Sylvester Equation with Xi Satisfies Different Linear Constra
- Matrices and Division by Zero z/0 = 0
- Jordan Γ*-Derivation on Semiprime Γ-Ring M with Involution
- Two Nonzero Component Lemma and Matrix Trigonometry
- Using Row Reduced Echelon Form in Balancing Chemical Equations
- Tight Monomials with t-Value ≤ 9 for Quantum Group of Type D4
- Minimum Covering Randić Energy of a Graph
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- Using Row Reduced Echelon Form in Balancing Chemical Equations
- Minimum Covering Randić Energy of a Graph
- A Note on the Inclusion Sets for Tensors
- A General Hermitian Nonnegative-Definite Solution to the Matrix Equation AXB = C
- Jordan Γ*-Derivation on Semiprime Γ-Ring M with Involution
- Matrices and Division by Zero z/0 = 0
- On Characterization of Poised Nodes for a Space of Bivariate Functions
- Pseudo Laguerre Matrix Polynomials, Operational Identities and Quasi-Monomiality
