Enantiomeric Separation of S-Epichlorohydrin and R-Epichlorohydrin by Capillary Gas Chromatography w
Vol.07No.11(2016), Article ID:71672,13 pages
10.4236/ajac.2016.711069
Cholleti Vijay Kumar1,2*, Pavan Kumar Vasa3, Y. Ravindra Kumar1, Pasula Aparna2, Padi Pratyusha1
1Dr. Reddy’s Laboratories Ltd. Active Pharmaceutical Ingredients, IPDO, Hyderabad, India
2Department of Chemistry, J.N.T. University, Hyderabad, India
3Department of Pediatrics, SUNY Stony Brook Medical Center, Stony Brook, New York, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 8, 2016; Accepted: October 28, 2016; Published: October 31, 2016
ABSTRACT
The aim of this study was to develop a simple and derivatization free method for the Quantification of S-Epichlorohydrin in R-Epichlorohydrin by using a gas chromatography coupled with flame ionization detector (FID). Enantiopure epichlorohydrin was a valuable epoxide key starting material for preparing optically active Rivaroxaban. The enantiomeric separations of S-Epichlorohydrin and R-Epichlorohydrin were achieved on Gamaa-Dex-225 (30 meters × 0.25 mm I.D, 0.25 µm) column with a total run time of 30 min. Nitrogen was used as a carrier gas with constant pressure 25.0 psi. The critical experimental parameters such as, column selection, flow rate, injection volume and diluent were studied and optimized. Excellent correlation coeffient between peak responses and concentrations was >0.9998. The recoveries of S-Epichlorohydrin spiked in R-Epichlorohydrin were in the range from 98.2% to 102.8%. Limit of quantitation for S-Epichlorohydrin was sufficiently lower than limits specified by ICH. The method has validated as per International Conference on Harmonization (ICH) guidelines. A precise, accurate, linear and robust Gas Chromatography method was developed for the quantification of S-Epichlorohydrin in R-Epichlorohydrin for Rivaroxaban.
Keywords:
S-Epichlorohydrin, R-Epichlorohydrin, Method Development, ICH Guidelines, Method Validation, Gas Chromatography

1. Introduction
Separation of the enantiomers of chiral drugs has become an important issue in analytical chemistry in recent years, because of differences in the biological activity and pharmacokinetic properties of drug enantiomers [1] [2] . Epichlorohydrin is an organochlorine compound and it is a chiral molecule and exists as R-Epichlorohydrin and S-Epichlorohydrin mentioned in Figure 1(a) and Figure 1(b). Epichlorohydrin is a versatile precursor in the synthesis of many organic compounds. Epichlorohydrin is a colorless, volatile and highly reactive liquid. It is soluble in most organic solvents and slightly soluble in petroleum hydrocarbons and in water [3] . Epichlorohydrin (1-chloro- 2,3-epoxypropane) was used mainly for the manufacture of pharmaceutical products, glycerol, unmodified epoxy resins and to a lesser extent elastomers, water-treatment resins, surfactants, ion exchange resins, plasticizers, dyestuffs, oil emulsifiers, lubricants, and adhesives [4] . Epichlorohydrin has been classified as a probable carcinogen (group A) to human by the International Agency for Research on Cancer (IARC) [5] .
Rivaroxaban is chemically 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-orpholinyl) phenyl]-1,3-oxzolidin-5-yl}methyl)-2-thiophene-carboxamide with molecular formula C19H18ClN3O5S. Rivaroxaban was used for potent anticoagulant and antithrombotic effects [6] [7] . Rivaroxaban was approved by US Food and Drug Administration (FDA). Gas chromatography is the most commonly used technology for the analysis of Epichlorohydrin. From the literature review there were few analytical methods which have been reported for Epichlorohydrin such as GC methods [8] [9] [10] [11] [12] and GC-MS methods [13] [14] . There was no reported method for the determination of S-Epichlorohydrin in R-Epichlorohydrin by gas chromatographic method for Rivaroxaban. The major objective of the present work is to develop a simple and robust GC method for determination of S-Epichlorohydrin in R-Epichlorohydrin for Rivaroxaban. Hence, a reproducible gas chromatography with FID detector method was developed for the quantitative determination of S-Epichlorohydrin in R-Epichlorohydrin. This method was successfully validated according to the International Conference Harmonization (ICH) guidelines (Validation of Analytical Procedures: Test and Methodology Q2).
2. Experimental
2.1. Materials and Reagents
R-Epichlorohydrin and S-Epichlorohydrin were purchased from Sigma-Aldrich Dich

 (a) (b)
(a) (b)
Figure 1. (a) Structures of R-Epichlorohydrin; (b) Structures of S-Epichlorohydrin.
loromethane was purchased from Merck, Germany.
2.2. Instruments and Software
A calibrated electronic single pan balance Mettler Toledo. All analysis performed on Agilent 6890 and 7890 modules equipped with FID detectors. Empower-3 software was used for signal monitoring and data processing. Microsoft Excel 2007 was used for analysis of validation results.
2.3. Chromatographic Conditions
The method was developed by using Gamaa-Dex-225 (30 meters × 0.25 mm I.D, 0.25 µm) column. The separation was achieved using an isothermal oven program 50˚C for 30 min. The injector temperature was maintained at 250˚C. Nitrogen was used as a carrier gas with constant pressure 25.0 psi. The detector temperature was maintained at 250˚C. The injection volume was 1.0 μL. Split ratio 1:50 and Runtime was 30.0 min.
Dichloromethane was used as diluent during the standard and test samples preparation. Chromatograms were summarized in Figures 2(a)-(d).
2.4. Preparation of Solutions
2.4.1. Preparation of S-Epichlorohydrin Stock Solution
Transfer accurately 0.1 mL of S-Epichlohydrin standard in 50 mL volumetric flask, containing 10.0 mL diluent and made up to volume with diluent.
2.4.2. Preparation of System Suitability Solution
Transfer accurately 1.0 mL of standard in 50 mL volumetric flask, containing 10.0 mL diluent and to this add accurately 0.5 mL of above S-Epichlorohydrin stock solution and made up to volume with diluent.
2.4.3. Preparation of Standard Solution
Transfer accurately 1.0 mL of standard in 50 mL volumetric flask, containing 10.0 mL diluent and made up to volume with diluent.
2.4.4. Sample Solution Preparation
Transfer accurately 1.0 mL of test sample in 50 mL volumetric flask, containing 10.0 mL diluent and made up to volume with diluent.
3. Method Validation
The method has been validated by GC as per ICH guidelines [15] . The method was validated for the following parameters: Precision, Linearity, Accuracy, Robustness, Solution stability, Limit of Detection, Limit of Quantification and Ruggedness.
3.1. Precision
The precision of the method was verified by repeatability and by intermediate preci- sion. Repeatability of the method was checked by (Agilent 7890 module equipped with
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e) (f)
(f)
Figure 2. (a) Typical chromatograms of blank; (b) Typical chromatograms of system suitability; (c) Typical chromatograms of R-Epichlorohydrin sample; (d) Typical chromatograms of R-Epi- chlorohydrin sample spiked at specification level; (e) Typical chromatograms of LOQ; (f) R-Epi- chlorohydrin sample spiked at LOQ level.
FID detector) injecting six individual preparations of R-Epichlorohydrin sample spiked with 0.10% of S-Epichlorohydrin (0.10% of S-Epichlorohydrin isomer with respect to 20 μL/mL R-Epichlorohydrin). The RSD of peak area for S-Epichlorohydrin was calculated. The intermediate precision of the method was also evaluated using different analysts, different instruments and different columns and performing the analysis on three different days.
3.2. Accuracy
For determination of accuracy of method recovery study was carried out by analyzing the spiked samples. Known amount of S-Epichlorohydrin was spiked in triplicate at three different concentration levels of 0.01, 0.02 and 0.03 μL/mL (50%, 100% and 150% of the analyte concentration i.e. 20 μL/mL) to the drug product. The % recoveries for S- Epichlorohydrin was calculated based on mentioned in Equation (1).
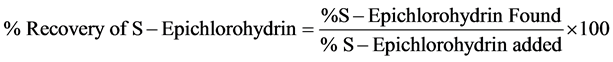 (1)
(1)
3.3. Linearity
To establish linearity of the method was prepared by diluting stock solution to the required concentration. The solutions were prepared at seven concentration levels from LOQ to 150% of the specification level (LOQ, 0.025, 0.050, 0.075, 0.10, 0.125and 0.150%) with respect to the normal sample concentration (20 μL/mL). The correlation coefficients, slopes and Y-intercepts of the calibration curve were determined.
3.4. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The LOD and LOQ for S-Epichlorohydrin were determined at a signal-to-noise ratio of 3:1 and 10:1, respectively, by injecting a series of dilute solutions with known concentrations. Precision study was also carried out at the LOQ level by injecting six (n = 6) individual preparations and calculating the RSD of the area of S-Epichlorohydrin.
3.5. Robustness
The robustness of an analytical procedure is a measure of its capability to remain unaltered by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage.
To determine the robustness of the method, the chromatographic conditions were deliberately changed and relative standard deviation of the S-Epichlorohydrin peak was evaluated. As the flow rate was 25 psi to study the effect of flow rate on relative standard deviation of the S-Epichlorohydrin peak, the flow rate was changed to 20 psi and 30 psi. The effects of the column oven temperature were studied at 45˚C and 55˚C instead of 50˚C.
3.6. Solution Stability
The stability of S-Epichlorohydrin in R-Epichlorohydrin solution was determined by leaving test solutions of the sample and spiked solution in tightly capped volumetric flasks at room temperature for 24 hrs during which they were analysed at 12 hrs intervals.
4. Results
4.1. Method Development and Optimization
The main goal of method development was to achieve separation of S-Epichlorohydrin in R-Epichlorohydrin without derivatization. An understanding of the nature of the racemic Epichlorohydrin is the foremost prerequisite for successful method development in GC. Following were the stepwise strategies for the method development in our case.
4.1.1. Column Selection
The primary goal of column selection was to separate S-Epichlorohydrin and R-Epi- chlorohydrin from each other, which were used during the synthesis of Rivaroxaban. As part of method development screened various columns, namely Chiral GTA (30 meters × 0.25 mm I.D, 0.12 µm) and Chiralsil (30 meters × 0.25 mm I.D, 0.25 µm) were employed but no adequate separation was found with above columns. After careful screening of columns, it was observed that Gamaa-Dex-225 (30 meters × 0.25 mm I.D, 0.25 µm) column provides better resolution between S-Epichlorohydrin and R-Epich- lorohydrin and it showed good system suitability parameters.
4.1.2. Flow Rate
As the flow rate increase, the viscosity of carrier gas decreased and velocity increased. Check the flow rate from 6 psi to 40 psi. At 6 psi, the retention time was very high and runtime is long, poor separation was observed at 40 psi. 25 psi was selected as finalized flow rate.
4.1.3. Selection of Diluent
Diluent selection study was conducted for the analysis of R-Epichlorohydrin and S- Epichlorohydrin. Four diluents had been tried N-methyl-2-pyrrolidone, Dimethyl formamide, Dimethyl imidazolidine and Dichloromethane. Dichloromethane was finalized as diluent because of no interference at the S-Epichlorohydrin and R-Epichlorohy- drin peaks.
4.1.4. Injection Volume
The effect of injection volume on the quantification of the S-Epichlorohydrin and R- Epichlorohydrin were investigated by injecting volume between 0.5 μL to 2 μL of the standard solution. The results show that the peak widths of S-Epichlorohydrin and R- Epichlorohydrin were independent of injection volume within the tested range.
System suitability results are shown in Table 1 and Figures 3(a)-(c).
5. Method Validation
5.1. Precision
The % RSD for the content of S-Epichlorohydrin in the method precision was found to be less than 1.1. The % RSD for the content of S-Epichlorohydrin in the intermediate precision (Ruggedness) was found to be less than 2.2 (Table 2). The results confirmed the high precision of the developed GC method.
5.2. Limit of Detection and Quantification
The obtained limit of detection and limit of quantification, precision and accuracy at limit of quantification values are given in Table 2 and Figure 2(e), Figure 2(f).
Table 1. System suitability parameters.
aRelative retention times (RRT) were calculated against the retention time (RT) of R-Epichlorohydrin. bRelative standard deviation. cMean ± SD.
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Method development chromatogram (a) method development chromatography conditions: chiralsil (30 m × 0.25 mm I.D, 0.25 µm) oven temperature 50˚C for 30 minutes. Resolution between S-Epichlorohydrin and R-Epichlorohydrin was 0.9. Method development chromatogram (b) method development chromatography conditions: chiral gta (30 m × 0.25 mm I.D, 0.12 µm) oven temperature 50˚C for 30 minutes. Resolution between S-Epichlorohydrin and R-Epichlo- rohydrin was 1.2 method development chromatogram (c) method development chromatography conditions: gamaa-dex-225 (30 meters × 0.25 mm I.D, 0.25 µm) oven temperature 50˚C for 30 minutes. Resolution between S-Epichlorohydrin and R-Epichlorohydrin was 4.3.
5.3. Accuracy
Individual and average recoveries of three preparations and at three concentrations for S-Epichlorohydrin were within 100% ± 5% results shown in Table 2 & Table 3.
Table 2. Summarized data of method validation.
5.4. Linearity
The calibration curve was drawn by plotting the peak area against the concentration. The correlation coefficient (r2) obtained was ˃0.9998. The % Y-intercept with respect to response at 100% level was ˃± 5%. The results for the Correlation coefficient (r2) and % Y-intercept with respect to response at 100% level were shown in Table 4 & Table 5. The results demonstrate that an excellent correlation existed between the peak area and concentration of S-Epichlorohydrin.
5.5. Robustness
In all the deliberately varied chromatographic conditions, no effect on the Relative standard deviation of the S-Epichlorohydrin peak (Table 2) the method was more robust within the normal operating range, i.e., column oven temperature, 50˚C ± 5˚C and flow rate, 25 ± 5 psi, demonstrating the robustness of the method results shown in Table 2.
Table 3. S-Epichlorohydrin accuracy and % RSD at 50%, 100% and 150% level.
Table 4. S-Epichlorohydrin linearity.
Table 5. S-Epichlorohydrin ANOVA.
5.6. Solution Stability
No significant change in the amounts of S-Epichlorohydrin was observed during solution stability experiments. The results from solution stability experiments confirmed that sample and spiked solutions were stable for up to 24 hrs results shown in Table 6.
6. Discussion
Based on the results, the successful separation of S-Epichlorohydrin and R-Epichloro- hydrin from each other. All the validated parameters were found to be within limits. System suitability for 6 injections % RSD was found to be NMT 0.54%. Precision at LOQ, 100% and 150% were found to be NMT 3.29%, Accuracy at LOQ, 50% 100% and 150% were found to be 98.2% to 102.8%. Linearity was performed from LOQ to 150% and graph obtained was linear showing correlation coefficient >0.9998.
Table 6. S-Epichlorohydrin solution stability.
7. Conclusion
A simple gas chromatographic method was developed and validated for the quantitative S-Epichlorohydrin in R-Epichlorohydrin for Rivaroxaban. S-Epichlorohydrin and R- Epichlorohydrin were well separated from each other, indicating that the developed GC method was specific. The method validation data showed satisfactory results for all tested method parameters. This simple GC method is precise, accurate, linear and rugged. Hence, it is proved that developed method can be used for routine testing in quality control laboratories for estimation S-Epichlorohydrin in R-Epichlorohydrin for Rivaroxaban. The method is user-friendly and robust to operate.
Acknowledgements
The authors wish to thank the management of Dr. Reddy’s Laboratories Ltd. for supporting this work. Co-operation from colleagues of Research & Development and Analytical Research & Development of Dr. Reddy’s Laboratories Ltd. is appreciated.
Cite this paper
Kumar, C.V., Vasa, P.K., Kumar, Y.R., Aparna, P. and Pratyusha, P. (2016) Enantiomeric Separation of S-Epichlorohydrin and R-Epichlo- rohydrin by Capillary Gas Chromatography with FID Detector. American Journal of Analytical Chemistry, 7, 772-784. http://dx.doi.org/10.4236/ajac.2016.711069
References
- 1. Lehmann, R., Voelter, W. and Liebich, H.M. (1997) Capillary Electrophoresis in Clinical Chemistry. Journal of Chromatography B, 697, 3-35.
http://dx.doi.org/10.1016/S0378-4347(97)00183-7 - 2. Naylor, S., Benson, L.M. and Tomlinson, A.J. (1996) Application of Capillary Electrophoresis and Related Techniques to Drug Metabolism Studies. Journal of Chromatography A, 735, 415-438.
http://dx.doi.org/10.1016/0021-9673(96)00068-4 - 3. United States Environmental Protection Agency, Office of Air Quality Planning and Standards (1984) Final Report, EPA-450/4-84-007j.
- 4. World Health Organization (1992) Chemical Review: Epichlorohydrin. Dangerous Properties of Industrial Materials Report, 12, 150-170.
- 5. International Agency for Research on Cancer (1999) Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Monographs on the Valuation of Carcinogenic Risk to Humans, 71, 603.
- 6. Susanne, R., Alexander, S. and Jens, P. (2005) Discovery of Novel Antithrombotic Agent BAY 59-7939. Journal of Medicinal Chemistry, 48, 5900-5908.
- 7. Perzborn, E., Strassburger, J. and Wilmen, A. (2005) In Vitro and In Vivo Studies of BAY 59-7939. Journal of Thrombosis and Haemostasis, 3, 514-521.
http://dx.doi.org/10.1111/j.1538-7836.2005.01166.x - 8. Lasa, M., Garcia, R. and Millán, E. (2006) A Convenient Method for Epichlorohydrin Determination in Water Using Headspace-Solid-Phase Micro Extraction and Gas Chromatography. Journal of Chromatographic Science, 44, 438-443.
http://dx.doi.org/10.1093/chromsci/44.7.438 - 9. Shaik, J.V., Ganduri, R.B. and Sait, S. (2011) Estimation of Epichlorohydrin Content in Pharmaceutical Drug Substances by Capillary Gas Chromatography with Flame Ionisation Detection. Journal of Chemical and Pharmaceutical Research, 3, 392-399.
- 10. Kadiyala, R.V., Kothapalli, P.K., Peddolla, M.R., Rajput, P., Sharma, H.K., Budeti, S.R., Gandham, H. and Nowduri, A. (2014) Development and Validation of a Gas Chromatography Method for the Trace Level Determination of Allylamine in Sevelamer Hydrochloride and Sevelamer Carbonate Drug Substances. Scientia Pharmaceutica, 82, 117-128.
http://dx.doi.org/10.3797/scipharm.1309-12 - 11. Karthikeyan, K., Arularasu, G.T., Perumalsamy, D. and Chandrasekara, P.K. (2010) Determination of Residual Epichlorohydrin in Sevelamer Hydrochloride by Static Headspace Gas Chromatography with Flame Ionization Detection. Scientia Pharmaceutica, 78, 835-846.
http://dx.doi.org/10.3797/scipharm.1007-20 - 12. Neu, H.J. and Sprenger, R. (1997) Trace Analysis of Epichlorohydrin in Water Samples. Fresenius’ Journal of Analytical Chemistry, 359, 285-287.
http://dx.doi.org/10.1007/s002160050574 - 13. Sung, J.H., Lee, Y.J. and Park, H.J. (2008) New Method for Determination of Epichlorohydrin in Epoxy-Coated Cans by Oxolane Derivatization and Gas Chromatography-Mass Spectrometry. Journal of Chromatography A, 1201, 100-105.
http://dx.doi.org/10.1016/j.chroma.2008.06.008 - 14. Loda, C., Bernabe, E., Nicoletti, A., Bacchi, S. and Dams, R. (2011) Determination of Epichlorohydrin in Active Pharmaceutical Ingredients by Gas Chromatography-Mass Spectrometry. Organic Process Research & Development, 15, 1388-1391.
http://dx.doi.org/10.1021/op200203t - 15. ICH Q2 (R1) (2005) Validation of Analytical Procedures: Text and Methodology.
上一篇:Identification, Isolation and 下一篇:Characterization of Lignin bef
最新文章NEWS
- Identification, Isolation and Structure Confirmation of Forced Degradation Products of Sofosbuvir
- Enantiomeric Separation of S-Epichlorohydrin and R-Epichlorohydrin by Capillary Gas Chromatography w
- Characterization of Lignin before and after Exposure to the Gastrointestinal Tract of Ruminants
- Sensitive Determination of Metal Ions in Drinking Water by Capillary Electrophoresis Coupled with Co
- Characterization of Urban Soil with SEM-EDX
- Alterations in Low-Z Elements Distribution in Heart Tissue after Treatments to Breast Cancer Using L
- Multivariate Optimization of Volatile Compounds Extraction in Chardonnay Wine by Headspace-Solid Pha
- Research of Anti-Cancer Components in Traditional Chinese Medicine on Hollow Fibre Cell Fishing and
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- Derivative Spectrophotometric and Isocratic High Performance Liquid Chromatographic Methods for Simu
- Volatile Organic Compounds in Crude Coconut and Petroleum Oils in Nigeria
- A Proton Nuclear Magnetic Resonance (1H NMR) Investigation of NaCl-Induced Phase Separation of Aceto
- An Application of Gas Chromatography-Mass Spectrometry (GC-MS) Fast Automated Scan/SIM Type (FASST)
- Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Build
- Photodegradation of Binary Azo Dyes Using Core-Shell Fe3O4/SiO2/TiO2 Nanospheres
- Preliminary Phytochemical Content and Antidiabetic Potential Investigations of Panda oleosa (Pierre)
- A Comparative Study of Heavy Metal Concentration in Different Layers of Tannery Vicinity Soil and Ne

