Analysis of Bacterial Cellulose/Ionic Liquid MWCNTs via Cyclic Voltammetry
Vol.06No.01(2016), Article ID:62731,9 pages
10.4236/aces.2016.61004
Seyed Morteza Zendehbad1, Guang Yang2
1College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China
2National Engineering Research Center for Nano-Medicine, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 December 2015; accepted 11 January 2016; published 14 January 2016
ABSTRACT
Cyclic voltammetry based on an electrochemical technique is one of the current methods that measure the developments of the electrochemical properties in biomaterial samples under conditions. Biomaterial structure was changed by conductive material while these materials caused a connective network in whole of them and was able to transfer electrons inside of biomaterials. These changes in physical and chemical properties are investigated by analysis tools such as cyclic voltammetry (CV), X-radiation (XRF) and Ultraviolet-visible spectroscopy (UV-Vis). Bacterial cellulose is biodegradable, biosynthesis of A. xylinum which is a three-dimensional nano-network structure with a distinct tunnel and pore structure. In this study, the composite process produced electrically conducting bacterial cellulose pellicles containing well-dispersed and embedded multi-walled carbon nanotubes (MWCNTs) Ionic liquids (ILs), as observed in cyclic voltammetry (CV). For this purpose, we used a special tool, called OriginLab which is an industry-leading scientific graphing and data analysis software. The cyclic voltammetry graph presents the behavior of this composite which consists of a relationship between CNT dispersion, conductivity rate and changes in bacterial cellulose structure. The electrical conductivity of the cellulose/MWCNT composite was found different with respect to CNT dispersion. It was found that the incorporation process was a useful method not only for dispersing MWCNTs-ILs in an ultrafine fibrous network structure, but also for enhancing the electrical conductivity of the polymeric membranes.
Keywords:
Electrical Conductivity, Ionic Liquid (IL), Multi-Walled Carbon Nanotubes (MWCNTs), Bacterial Cellulose (BC), Cyclic Voltammetry (CV), OriginLab

1. Introduction
Bacterial cellulose (BC) is provided by some categories of acetic acid bacteria [1] - [3] . BC properties are including high crystallinity and mechanical strength, important porosity and water-holding capacity. Corresponding to these properties, it’s an extremely functional biomaterial in varied different industrial processes such as in food and medical applications [1] - [5] .
As experience of most related radicals declared, there are two methods to generate BC, namely stationary and agitated culture. Mainly, the macroscopic morphology of Bacterial Cellulose varies according to the cultural approaches [6] [7] . Typically, stationary culture results in the accumulation of a gelatinous membrane of cellulose on the surface of the medium; and in agitated culture, cellulose is synthesized in deep media in the form of irregular masses, fibrous suspensions, pellets [6] [8] . Overall, stationary culture conditions investigated successfully. However, the agitated culture can be extremely suitable means for economic balance but there are numerous problems such as non-Newtonian behavior during mixing of BC, proper oxygen supply for the agitated culture of Acetobacter strains [5] [8] [9] .
Moreover, some microstructure is dependent on cultural conditions such as crystallinity, crystalline polymorphism, crystallite size, cellulose Iα content and mechanical properties of BC. In static culture, the effects of water-soluble agents and polysaccharides in culture media on the aggregation and crystallization of BC microfibrils were intensively studied [2] [10] . The outcomes uncovered that the additives in stationary culture affect on the cellulose behavior such as the crystallite size, crystallinity index and cellulose (Iα) content, postpone the aggregation of cellulose microfibrils and restrict the crystallization of cellulose microfibrils. While silica sol is inserted into the stationary growth medium, corresponding silica adjusts the elasticity and strength of BC [9] [11] .
Specifically, cellulose from a bacterial source presents higher crystallinity and has noticeable advantages in comparison with other sources. These benefits contain high purity and excellent surface area whenever compared with plant cellulose. Figure 1 indicates the bacterial cellulose under microscopy investigation. The scanning electron microscopy images of BC pellicles show the planar extensively cross-linked structure and the relatively weakly cross-linked layers in the thickness direction [10] [12] [13] .
Polymer science uses ionic liquids (ILs) as polymerization media in various models of polymerization pro- cesses, involving conventional free radical polymerization [14] [15] . Moreover, it’s used for living/controlling fundamental polymerizations, for instance: atom transfers radical polymerizations (ATRP), reversible addition- fragmentation transfer (RAFT), as well as in ionic and coordination polymerizations [15] [16] .
Overall, ionic liquids are the important effects on varying categories of polymerizations.
It is remarkably significant that ionic polymerizations are developed in IL solvents. Therefore, the atom trans- fers radical polymerization (ATRP) catalysts composed to ILS to cause them more comfortably and recoverability in exiting polymerizations [15] [16] . Furthermore, the number of polymerizable ILs is steadily expanding. Also ILs polymers of polymerizable ionic liquid monomers have been constructed as exotic polyelectrolytes. ILs are tended to use as plasticizers of polymers in some categorize and as key components in novel classes of polymer gels [17] - [19] .

Figure 1. The scanning electron microscopy images of BC pellicles.
Although an Ionic liquid’s characteristic is an excellent ionic conductivity, depends on its decomposition temperature. According to this property, they are significant in electrolyte matrixes [15] [20] [21] . However, eliminating leakage’s outlook mentions that solid or quasi-solid ion-conductive electrolytes get better position compares with fluidic materials. Hence it is advantageous to convert IL-based electrolyte solutions into a solid or quasi-solid form. Recently, ILs-Gelation has been the most studied issues and supported both physical and chemical gelations [18] [19] . Among these methods have been used for the gelation of ILs, inorganic nanoparticles, Low-molecular weight compounds, single-walled carbon nanotubes are successful [22] .
Undoubtedly, an ionicliquid-assisted method is a known and effective approach. This powerful method improved the fabrication of metal nanoparticles based on multi-walled carbon nanotubes (MWCNTs) [23] [24] . Technically, small changes in the amount of ionic liquids in the reaction solution influences to a production of well-crystallized metallic nanoparticles. This product has some properties such as tunable diameter and narrow size distribution, uniformly dispersed on MWCNTs [23] [25] .
The MWCNT-IL is obtained through a three-step procedure. The first step cuts highlyentangled, long MWCNTs into shorter, open-ended pipes, and functionalizes them with polar hydrophilic groups (COOH, C=O, OH) on the surface by acid oxidation and sonication. In the second step, reaction occurs with a polysiloxane quaternary ammonium salt (DC5700) through bonding with the OH or COOH group [23] [26] .
During the third step, the resulting structure reacts with sulfonate salts via a cation-anion process to form a MWCNT-IL. Thus, our product is essentially different from the reported amphiphilic derivatives prepared by a functional CNT-amine reaction method.
In summary, 100 - 500-nm-long MWCNTs with an ionic liquid ACHTUNGTRENUNG like behavior and an excellent thermal stability were obtained. An approximately 6.5-nm-thick shell model with an ionic material, perpendicular bilayer structure on the CNT surface was verified. This finding will offer a new practical method to process “solid CNTs” or polymer-CNT composites, such as macromolecules, colloids, or even solutions [27] [28] . In addition, the process ability can be improved by cutting MWCNTs into shorter nanotubes and enhancing the organic content of the derivatives. Our further studies will focus on the fluidity mechanism supported by the ionic bilayer around the CNTs and the process ability values for MWCNT-IL-polymer composites.
2. Experimental Section
2.1. Preparation of Bacterial Cellulose
Gluconacetobacter xylinum (ATCC53582) was used for the biosynthesis of BC. The bacterium was cultured in a Hestrin and Schramm (HS) medium, which was composed of 2% (wt) glucose, 0.5% (wt) yeast extract, 0.5% (wt) peptone, 0.27% (wt) disodium phosphate, and 0.15% (wt) citric acid. After incubating statically for 14 days at 26˚C, the BC membranes were dipped into distilled water for 2 days, and then keep in a 1 wt% NaOH solution for 30 min to eliminate bacteria and proteins (Figure 2). Afterwards, the BC membranes were purified by being washed in distilled water several times, and were then stored in distilled water at 4˚C [5] .



Figure 2. Bacterial cellulose 1, 7, 14 days (left to right), the thickness of bacterial cellulose depends on growth time.
2.2. Preparation of BC/MWCNTs and BC/MWCNT-ILs
The commercial MWCNTs used for this experiment has about 40 - 60 nm diameter, 300 - 600 nm length, a specific density of 140 to 300 g/dm (3) which is carbon content of above 80%. Typically, commercial MWCNTs (2 g) were sonicated for 2 h in a warm bath followed by refluxing in a mixture of concentrated sulfuric and nitric acids (3:1, 98% and 65%, 350 mL) for 12 h. This procedure gave a high-concentration black suspension. The suspension was diluted with deionized water (total volume 1000 mL) and filtered through nylon membrane filters (pore size 0.22 mm, diameter 47 mm). The remaining solid was washed repeatedly with water to adjust the pH value (pH 5 - 7). DC5700 in methanol (40%, Gelest) was added to the suspension and the mixture was sonicated for 2 h. Then the solid (DC5700-grafted MWCNTs) was carefully rinsed three times with water and methanol until there was no foam left, re-suspended in methanol, and dried at 70˚C.
The corresponding MWCNT-IL was prepared by reaction with a solution of C9H19C6H4O ACHTU- NGTRENUNG (CH2CH2O)10∙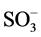 K+ (55 mL, 10.5% w/v, Aldrich) in water at 70˚C for 24 h. Following removal of the solution, the material was washed several times with water and methanol and dried at 70˚C for two days (yield 5 g). Figure 3 shows the BC was immersed into MWCNTs solution (0.02 wt%, 0.05 wt%) and MWCNT-ILs solution (0.04, 0.02, 0.005, and 0.002 wt%). Ultrasound was then applied to the MWCNT dispersion using an ultrasonic generator about 2 h, then stirring about 48 h.
K+ (55 mL, 10.5% w/v, Aldrich) in water at 70˚C for 24 h. Following removal of the solution, the material was washed several times with water and methanol and dried at 70˚C for two days (yield 5 g). Figure 3 shows the BC was immersed into MWCNTs solution (0.02 wt%, 0.05 wt%) and MWCNT-ILs solution (0.04, 0.02, 0.005, and 0.002 wt%). Ultrasound was then applied to the MWCNT dispersion using an ultrasonic generator about 2 h, then stirring about 48 h.
3. Results and Discussion
In above, we mentioned the method of bacterial cellulose preparation. The BC was immersed into multi-walled carbon nano tubes, which are combined with ionic liquids in different solutions as figure three presents. Ultrasound was then applied to the MWCNT dispersion using an ultrasonic generator about 2 h, next stirring about 48 hours. The following step was drying membranes and kept on stable form. Virtually in Figure 4, there are two samples after drying in at 120˚C and 1 - 2 MPa. The different MWCNTs dispersions are clearly visible while the black shadow tends from the center to the edge. However, we needed investigating deeply; therefore, we used cyclic voltammetry to study this property.
Cyclic voltammetry or CV is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time like linear sweep voltammetry. Cyclic voltammetry takes the experiment a step further than linear sweep voltammetry which ends when it reaches a set potential. When cyclic voltammetry reaches a set potential, the working electrode’s potential ramp is inverted. This inversion can happen multiple times during a single experiment. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. For this purpose, we

Figure 3. Schematic diagram of the process of incorporating the nanotubes into the water- swollen bacterial cellulose pellicle [10] .
prepared the cyclic voltammetry of different electrodes in 10 mM pH 7.4 PBS containing 5.0 mM K3(Fe(CN)6) and 1.0 M KCl. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution.
Principally, the sensitivity of a cell impedance sensor corresponds to the response of a redox probe at the sensor. Potassium ferricyanide (K3(Fe(CN)6)) was chosen as a probe to evaluate the performance of the proposed electrodes. All collected data submitted to OriginLab software to analyses and draw the related graphs. The results are present in Figure 4 and Figure 5 which are covered some parts of data and exhibit several voltammogram curves. Figure 4 exhibited typical electrochemical responses of BC-MWCNTS where the samples are distinctive MWCNTs dispersion 0.02 wt% and 0.05 wt% voltammogram curves.

Figure 4. Cyclic voltammetry of different electrodes in 10 mM pH 7.4 PBS containing 5.0 mM K3(Fe(CN)6) and 1.0 M KCl. It shows relative curve to BC-MWCNTS 0.02 wt% and 0.05 wt%.

Figure 5. Cyclic voltammetry of different electrodes in 10 mM pH 7.4 PBS containing 5.0 mM K3(Fe(CN)6) and 1.0 M KCl. corresponding to different dispersion (0.04, 0.02, 0.005, and 0.002) wt%.
As we reverse the voltage scan, we still have a layer depleted of the oxidized species, but the surface concentration begins to rise and the electrical current decreases further. Finally, we reached a region where the anodic current begins to dominate (Figure 5). We achieved a peak negative current and afterwards the current will decrease in magnitude as the depletion layer for the reduced species increases. Also Figure 5 shows the voltammogram curves for BC-MWCNTs-IL corresponding to different dispersion (0.04, 0.02, 0.005, and 0.002) wt% in 0.1 M KCl + 1 mM K3Fe(CN)6 solution, respectively. As seen in Figure 5, depicting a cyclic voltammogram for the composite in 10 mM PBS electrolyte, the composite material also showed a high degree of electro-activ- ity. The CV of BC-MWCNTs-IL 0.04 wt% identified an oxidation peak at +230 mV (curve d) and the modified BC-MWCNTs-IL 0.02 wt% also showed an oxidation peak at +220 mV (curve c). It’s clear that “a, b” curves have a low degree of electro-activity because of the low dispersion of MWCNTs-IL combination by bacterial cellulose.
It was shown that, in the comparison between CVs obtained on the electrodes, the formation of BC- MWCNTs-IL film at the electrode decreased the redox peak currents greatly (curves d and c), while the incorporation of MWNTs in the BC film (curve a) resulted in great increase of the redox peak current in comparison with that of 0.002 wt%, and even higher than that of bare 0.02 wt% (Figure 6). However, as showed in


Figure 6. Zoom area of cyclic voltammetry of different electrodes in 10 mM pH 7.4 PBS containing 5.0 mM K3(Fe(CN)6) and 1.0 M KCl. corresponding to different dispersion (0.04, 0.02, 0.005, and 0.002) wt%.
Figure 6, zoom area, the peak value is increased relative to dispersion MWCNTs-IL where we draw the special peak value graph. In one side, this graph shows the behavior and effectiveness of MWCNTs dispersion and in the other side, it shows the rate of electro-activity.
4. Conclusion
Although a significant number of articles have examined the specific compounds but comparative evaluation method for testing the accuracy is low. In this paper, we attempted to present a descriptive and visual method to complete this topic. It was found that the incorporation process is a useful method not only for dispersing MWCNTs-IL and MWCNTs in an ultrafine fibrous network structure rather also for enhancing the electrical conductivity of the polymeric membranes. Moreover, we show that this component can use such as electrical elements because of high electro activity. Electrical elements are basically resistor, dielectric, wires and developmentally capacitor, transistor and professionally integrated circuit (IC), lab on a chip. Although we avoided long description about electrical elements but we presented the ability and capacity of BC/MWCNTs nanocomposites for using in this area.
Acknowledgements
We gratefully acknowledge use of facilities and instrumentation supported by the Huazhong University of Science and Technology (HUST) and College of Life Science and Technology. We thank Prof. Yang Guang for guide and help in experiments.
Cite this paper
Seyed MortezaZendehbad,GuangYang, (2016) Analysis of Bacterial Cellulose/Ionic Liquid MWCNTs via Cyclic Voltammetry. Advances in Chemical Engineering and Science,06,34-42. doi: 10.4236/aces.2016.61004
References
- 1. Fontana, J.D., de Souza, A.M., Fontana, C.K., Torriani, I.L., Moreschi, J.C., Gallotti, B.J., de Souza, S.J., Narcisco, G.P., Bichara, J.A. and Farah, L.F. (1990) Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Applied Biochemistry and Biotechnology, 24-25, 253-264.
http://dx.doi.org/10.1007/BF02920250 - 2. Hu, W., Chen, S., Yang, J., Li, Z. and Wang, H. (2014) Functionalized Bacterial Cellulose Derivatives and Nanocomposites. Carbohydrate Polymers, 101, 1043-1060.
http://dx.doi.org/10.1016/j.carbpol.2013.09.102 - 3. Zhu, C., Li, F., Zhou, X., Lin, L. and Zhang, T. (2014) Kombucha-Synthesized Bacterial Cellulose: Preparation, Characterization, and Biocompatibility Evaluation. Journal of Biomedical Materials Research Part A, 102, 1548-1557.
http://dx.doi.org/10.1002/jbm.a.34796 - 4. Kim, Y.-H., Park, S., Won, K., Kim, H.J. and Lee, S.H. (2013) Bacterial Cellulose-Carbon Nanotube Composite as a Biocompatible Electrode for the Direct Electron Transfer of Glucose Oxidase. Journal of Chemical Technology & Biotechnology, 88, 1067-1070.
http://dx.doi.org/10.1002/jctb.3939 - 5. Zendehbad, S.M. (2015) Controllable Fabrication of BC Based on Time Growth. International Journal of Materials Science and Applications, 4, 299-302.
- 6. Fink, H., Ahrenstedt, L., Bodin, A., Brumer, H., Gatenholm, P., Krettek, A. and Risberg, B. (2011) Bacterial Cellulose Modified with Xyloglucan Bearing the Adhesion Peptide RGD Promotes Endothelial Cell Adhesion and Metabolism— A Promising Modification for Vascular Grafts. Journal of Tissue Engineering and Regenerative Medicine, 5, 454-463.
http://dx.doi.org/10.1002/term.334 - 7. Chao, Y., Mitarai, M., Sugano, Y. and Shoda, M. (2001) Effect of Addition of Water-Soluble Polysaccharides on Bacterial Cellulose Production in a 50-L Airlift Reactor. Biotechnology Progress, 17, 781-785.
http://dx.doi.org/10.1021/bp010046b - 8. Yoon, S.H., Jin, H.-J., Kook, M.-C. and Pyun, Y.R. (2006) Electrically Conductive Bacterial Cellulose by Incorporation of Carbon Nanotubes. Biomacromolecules, 7, 1280-1284.
http://dx.doi.org/10.1021/bm050597g - 9. Yan, Z., Chen, S., Wang, H., Wang, B. and Jiang, J. (2008) Biosynthesis of Bacterial Cellulose/Multi-Walled Carbon Nanotubes in Agitated Culture. Carbohydrate Polymers, 74, 659-665.
http://dx.doi.org/10.1016/j.carbpol.2008.04.028 - 10. Shi, Z., Zang, S., Jiang, F., Huang, L., Lu, D., Ma, Y. and Yang, G. (2012) In Situ Nano-Assembly of Bacterial Cellulose-Polyaniline Composites. RSC Advances, 2, 1040-1046.
http://dx.doi.org/10.1039/C1RA00719J - 11. Yano, S., Maeda, H., Nakajima, M., Hagiwara, T. and Sawaguchi, T. (2008) Preparation and Mechanical Properties of Bacterial Cellulose Nanocomposites Loaded with Silica Nanoparticles. Cellulose, 15, 111-120.
http://dx.doi.org/10.1007/s10570-007-9152-x - 12. Nogi, M. and Yano, H. (2008) Transparent Nanocomposites Based on Cellulose Produced by Bacteria Offer Potential Innovation in the Electronics Device Industry. Advanced Materials, 20, 1849-1852.
http://doi.wiley.com/10.1002/adma.200702559
http://dx.doi.org/10.1002/adma.200702559 - 13. Wang, W., Zhang, T.-J., Zhang, D.-W., Li, H.-Y., Ma, Y.-R., Qi, L.-M., Zhou, Y.-L. and Zhang, X.-X. (2011) Amperometric Hydrogen Peroxide Biosensor Based on the Immobilization of Heme Proteins on Gold Nanoparticles-Bacteria Cellulose Nanofibers Nanocomposite. Talanta, 84, 71-77.
http://dx.doi.org/10.1016/j.talanta.2010.12.015 - 14. Marsh, K.N., Deev, A., Wu, A.C.-T., Tran, E. and Klamt, A. (2002) ChemInform Abstract: Room Temperature Ionic Liquids as Replacements for Conventional Solvents: A Review. ChemInform, 33, no.
- 15. Lu, J., Yan, F. and Texter, J. (2009) Advanced Applications of Ionic Liquids in Polymer Science. Progress in Polymer Science, 34, 431-448.
http://linkinghub.elsevier.com/retrieve/pii/S0079670008001226
http://dx.doi.org/10.1016/j.progpolymsci.2008.12.001 - 16. Sarbu, T. and Matyjaszewski, K. (2001) ATRP of Methyl Methacrylate in the Presence of Ionic Liquids with Ferrous and Cuprous Anions. Macromolecular Chemistry and Physics, 202, 3379-3391.
http://dx.doi.org/10.1002/1521-3935(20011101)202:17<3379::AID-MACP3379>3.0.CO;2-3 - 17. Ikeda, A., Sonoda, K., Ayabe, M., Tamaru, S.-I., Nakashima, T., Kimizuka, N. and Shinkai, S. (2001) Gelation of Ionic Liquids with a Low Molecular-Weight Gelator Showing Tgel above 100℃. Chemistry Letters, 30, 1154-1155.
http://dx.doi.org/10.1246/cl.2001.1154 - 18. Wang, P., Zakeeruddin, S.M., Comte, P., Exnar, I. and Grätzel, M. (2003) Gelation of Ionic Liquid-Based Electrolytes with Silica Nanoparticles for Quasi-Solid-State Dye-Sensitized Solar Cells. Journal of the American Chemical Society, 125, 1166-1167.
http://dx.doi.org/10.1021/ja029294+ - 19. Hanabusa, K., Fukui, H., Suzuki, M. and Shirai, H. (2005) Specialist Gelator for Ionic Liquids. Langmuir, 21, 10383-10390.
http://dx.doi.org/10.1021/la051323h - 20. Chen, Z., Li, F., Yang, H., Yi, T. and Huang, C. (2007) A Thermostable and Long-Term-Stable Ionic-Liquid-Based Gel Electrolyte for Efficient Dye-Sensitized Solar Cells. ChemPhysChem, 8, 1293-1297.
http://dx.doi.org/10.1002/cphc.200700116 - 21. Fukushima, T. and Aida, T. (2007) Ionic Liquids for Soft Functional Materials with Carbon Nanotubes. Chemistry—A European Journal, 13, 5048-5058.
http://dx.doi.org/10.1002/chem.200700554 - 22. Yu, P., Yan, J., Zhao, H., Su, L., Zhang, J. and Mao, L. (2008) Rational Functionalization of Carbon Nanotube/Ionic Liquid Bucky Gel with Dual Tailor-Made Electrocatalysts for Four-Electron Reduction of Oxygen. The Journal of Physical Chemistry C, 112, 2177-2182.
http://dx.doi.org/10.1021/jp709749j - 23. Chu, H., Shen, Y., Lin, L., Qin, X., Feng, G., Lin, Z., Wang, J., Liu, H. and Li, Y. (2010) Ionic-Liquid-Assisted Preparation of Carbon Nanotube-Supported Uniform Noble Metal Nanoparticles and Their Enhanced Catalytic Performance. Advanced Functional Materials, 20, 3747-3752.
http://dx.doi.org/10.1002/adfm.201001240 - 24. Lei, Y., Xiong, C., Dong, L., Guo, H., Su, X., Yao, J., You, Y., Tian, D. and Shang, X. (2007) Ionic Liquid of Ultralong Carbon Nanotubes. Small, 3, 1889-1893.
http://dx.doi.org/10.1002/smll.200700250 - 25. Zhang, Y., Shen, Y., Li, J., Niu, L., Dong, S. and Ivaska, A. (2005) Electrochemical Functionalization of Single-Walled Carbon Nanotubes in Large Quantities at a Room-Temperature Ionic Liquid Supported Three-Dimensional Network Electrode. Langmuir, 21, 4797-4800.
http://dx.doi.org/10.1021/la050026+ - 26. Saran, N., Parikh, K., Suh, D.-S., Muñoz, E., Kolla, H. and Manohar, S.K. (2004) Fabrication and Characterization of Thin Films of Single-Walled Carbon Nanotube Bundles on Flexible Plastic Substrates. Journal of the American Chemical Society, 126, 4462-4463.
http://dx.doi.org/10.1021/ja037273p - 27. Sun, Y.-P., Fu, K., Lin, Y. and Huang, W. (2002) Functionalized Carbon Nanotubes: Properties and Applications. Accounts of Chemical Research, 35, 1096-1104.
http://dx.doi.org/10.1021/ar010160v - 28. Yurekli, K., Mitchell, C.A. and Krishnamoorti, R. (2004) Small-Angle Neutron Scattering from Surfactant-Assisted Aqueous Dispersions of Carbon Nanotubes. Journal of the American Chemical Society, 126, 9902-9903.
http://dx.doi.org/10.1021/ja047451u
Abbreviations
BC: Bacterial cellulose;
G. xylinum: Gluconacetobacter xylinum;
A. xylinum: Acetobacter xylinum;
SEM: Scanning electron microscope;
AMT: Advanced microscopy techniques;
XRD: X-ray diffraction;
RST: Raman spectroscopy technique;
CV: Cyclic voltammetry;
XRF: X-radiation;
UV-Vis: Ultraviolet-visible spectroscopy;
OriginLab: Origin is an industry-leading scientific graphing and data analysis software.
上一篇:Solubility of Solids in Superc 下一篇:A Convective Thin Layer Drying
最新文章NEWS
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Solubility of Solids in Supercritical Fluids: The Mendez-Santiago Teja Model Revisited
- Solventless Organic Reactive Crystallization at Mild Conditions
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Luminescent Characteristic of Organic Compound-Containing Inorganic Crystal at Room Temperature
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Tunable Polymorphic Transformation Temperature
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- A Convective Thin Layer Drying Model with Shrinkage for Kent Mango Slices
- Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Optimisation of Decolourisation Conditions of Crude Shea (Vitellaria paradoxa Gaertner F) Butter: Ye
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Solvent Extraction of Citric Acid with Different Organic Phases
