NaBH4Sodalites, Synthesized by Modified Methods: (1) Autothermal Synthesis and (2) Crossover Reactio
Vol.07No.02(2017), Article ID:74538,17 pages
10.4236/aces.2017.72009
J.-Ch. Buhl
Institut für Mineralogie, Leibniz Universität Hannover, Hannover, Germany

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 4, 2017; Accepted: February 27, 2017; Published: March 2, 2017
ABSTRACT
NaBH4 sodalites were obtained by two new modified methods of crystalliza- tion: (1) autothermal synthesis and (2) crystallization with crossover from gel to melt flow in NaOH flux. Syntheses results were presented according to XRD, SEM and FTIR. Besides important features of both synthesis procedures product properties like crystal size and morphology were investigated. Spherical agglomerates of microcrystalline sodalite of composition Na7[AlSiO4]6BH4(H2O)2 were already observed after 4 h without any external heating by the autothermal procedure. Sodalites of the same average composition but in form of agglomerated nanoparticles are crystallized after very short times (2 h 30’) by the crossover reaction from gel to melt flow. Hydrogen release by heating was further studied for two selected samples with comparable composition from each synthesis procedure. Total hydrogen release by hydrolysis reaction with the internal cage water was found during heating of the autothermal product in synthetic air up to 550˚C. In contrast hydrogen release from the nanocrystalline sample of crossover synthesis was not completed when heated under the same conditions. These differences were discussed in terms of crystal size and an earlier loss of the internal water from the nanocrystals of the crossover synthesis.
Keywords:
Modified Synthesis, Autothermal Process, Combined Synthesis Procedures, Tailored Tetrahydroborate Sodalites

1. Introduction
Sodium-tetrahydroborate (NaBH4) is a representative material for hydrogen storage and contains 10.6 wt% H2 [1] but is a moisture sensitive compound. As a first model case for protecting this highly reactive salt from hydrolysis reaction, tetrahydroborate can be incorporated into the micropores of zeolite sodalite (SOD) [2] . The resulting 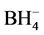 -enclathrated sodalite (ideal composition: Na8[AlSiO4]6(BH4)2) is also of interest to study the reaction behaviour of isolated BH4-anions during the release of hydrogen by thermal reactions [3] [4] [5] [6] . Even for the developments of new applications NaBH4 sodalite will attain more importance in future. The crystal structure of the mineral sodalite is known since decades [7] [8] [9] . As a member of the sodalite group the crystal structure of NaBH4 sodalite consists of a three-dimensional network of crosslinked TO4 tetrahedra (T = Si, Al) [2] . Polyhedral units in form of truncated octahedral cages (“toc-units”) in a space-filling array can be well distinguished as larger building blocks of the whole framework. A single BH4-anion is located within each toc unit, surrounded by four sodium cations, forming a [Na4BH4]3+-ion complex as complete cage filling. This ion complex is in fast rotational motion whereby the six-ring openings of the toc-unit are blocked by the sodium cations. Reactants like water molecules cannot enter the toc and the highly reactive and moisture sensitive BH4-anion is protected according this structural arrangement (the first synthesized Na4BH4 sodalite sample is still in perfect order since more than ten years [2] ).
-enclathrated sodalite (ideal composition: Na8[AlSiO4]6(BH4)2) is also of interest to study the reaction behaviour of isolated BH4-anions during the release of hydrogen by thermal reactions [3] [4] [5] [6] . Even for the developments of new applications NaBH4 sodalite will attain more importance in future. The crystal structure of the mineral sodalite is known since decades [7] [8] [9] . As a member of the sodalite group the crystal structure of NaBH4 sodalite consists of a three-dimensional network of crosslinked TO4 tetrahedra (T = Si, Al) [2] . Polyhedral units in form of truncated octahedral cages (“toc-units”) in a space-filling array can be well distinguished as larger building blocks of the whole framework. A single BH4-anion is located within each toc unit, surrounded by four sodium cations, forming a [Na4BH4]3+-ion complex as complete cage filling. This ion complex is in fast rotational motion whereby the six-ring openings of the toc-unit are blocked by the sodium cations. Reactants like water molecules cannot enter the toc and the highly reactive and moisture sensitive BH4-anion is protected according this structural arrangement (the first synthesized Na4BH4 sodalite sample is still in perfect order since more than ten years [2] ).
Up to now synthesis of Na8[AlSiO4]6(BH4)2 is exclusively performed under hydrothermal conditions, a method, general used in zeolite chemistry. This method is characterized by the presence of an excess amount of water and the need of autoclaves at elevated temperature. The successful insertion of the hydrothermal method year in and year out in zeolite chemistry caused some negligence of enhancements of common synthesis procedures. Thus future research on modified methods and alternative less energy consuming cheap procedures is essential to obtain new materials with tailored properties even under insertion of hydrolysis sensitive reagents.
The present investigation takes up this demand and presents a case study of tailored synthesis of NaBH4-sodalites according to two new enhanced crystallization techniques. The autothermal synthesis (1), first described for hydrosodalites in [10] and the low temperature crossover process from gel-like aqueous solution to melt flow (2), first introduced for halide sodalite in [11] . Both new enhanced methods will now be described in separate sections.
1) The autothermal synthesis
Here the thermal energy for the whole synthesis period is produced chemically within the reaction batch itself. A tailored exothermic process with high degree of energy transfer of the reaction enthalpy has to be applied. The hydrolysis of Al (∆H = −277 kJ/mol H2; .∆S = 26.2 J/K und ∆G = −284 KJ [12] ) is a suitable reaction for aluminosilicate sodalite synthesis. Besides providing of the whole heat energy for the synthesis process even the aluminate source for sodalite formation is produced under these aqueous alkaline conditions too, according to the reaction [13] :
 (1)
(1)
A temperature decrease during the reaction period is compensated by the chemical energy of the inserted strong alkaline solution. In [14] [15] it was shown that synthesis temperature and reaction time both can be remarkably reduced under insertion of high Na2O concentrations of 250 g/l for zeolite synthesis from aluminosilicate gels.
The autothermal process is tested for the first time in the present work for tailored syntheses in the tetrahydroborate sodalite system.
2) synthesis under conditions of crossover from aqueous gel-like solution to NaOH melt flow
The crossover reaction from aqueous gel solution to low temperature melt flow is demonstrated here as a further method for tailored synthesis of NaBH4- sodalite. The reaction mechanism based upon two distinctive steps, both ruled by controlled heating of a tailored educt mixture. In step one nucleation and early growth starts under the influence of water, released from the hydrated educts themselves during heating up. This early period is thus comparable with conditions of gel crystallization but under insertion of minimal amounts of water. In step two further crystal growth occurs under continuous shift of the conditions into a melt flux process at elevated temperature. Here the relatively low melt temperatures of 320˚C - 370˚C are sufficient, when a suitable flux component is added to the educts [11] .
Zeolite 13-X was selected as Si-Al-source that simultaneously also acts as “water provider” for the first step of the reaction. Furthermore NaOH granulate is added. NaOH is not only responsible to reach alkaline conditions for sodalites but also acts as fluxing agent at elevated temperature (Tmax.) in the second step of the whole process.
For a statement of the mechanism of this procedure one can consider the whole reaction as a crossover synthesis in (aqueous) gel-like solution followed by crystal growth in a melt as a one-pot process. The present work demonstrates this new preparation technique as a case study of insertion of a modified method for tailored synthesis in the NaBH4-sodalite system for the first time.
2. Experimental
-autothermal synthesis of NaBH4-sodalite
According to Equation (1) the NaOH concentration is a main synthesis parameter and four experiments with 4-, 8-, 12- and -16 M solutions were performed. Adequate portions of 6.4 - 25.6 g solid NaOH granulate (Merck 1.06467) were therefore filled into a 200 ml Teflon coated thermobeaker. 40 ml water and 5 ml sodium silicate solution (Merck 1.05621, consisting of 7.5% - 8.5% Na2O and 25.5% - 28.5% SiO2) were added. Additionally 1 g NaBH4 (Merck 806373) was admixed before 0.8 g aluminium grit (Riedel-de Haen St 615/8162) was carefully purred in. The thermobeaker was then closed by a cup, allowing the outlet of hydrogen produced during the rapidly starting strong exothermic reaction (see Equation (1)). A schematic view of the simple experimental arrangement is given in Figure 1.

Figure 1. Schematic view of the experimental procedure of the autothermal synthesis.
The intermediate product NaAlO2 and the sodium silicate solution react forming a zeolite precursor sludge according to Breck [16] :
 (2)
(2)
As a result of the rapidly rising temperature within the strong exothermic reaction system this process is overlapped by NaBH4-sodalite crystallization under inclusion of BH4 anions according to Equation (3):
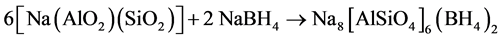 (3)
(3)
After a reaction period of 4 hours the products were washed (300 ml water) and dried over night on air to prevent any effects of higher temperature of drying in a cabinet dryer. The experimental conditions are summarized in Table 1.
-synthesis under conditions of crossover from aqueous gel-like solution to NaOH melt flow
A dry powder mixture of 200 mg zeolite 13-X (Fluka 69856), 50 mg NaOH granulate (Merck 1.06467) and 100 mg of NaBH4 (Merck 806373) was pressed into a pellet under a hydraulic press at 50 kN for 5 minutes, before heated under open conditions. A heating program RT → Tmax = 320˚C - 370˚C → RT was revealed for syntheses with 1.5 h heating up till Tmax. and 1.0 h holding time at Tmax. After this reaction period the dense as synthesized sample pellets were grinded in a mortar, before washed (100 ml water) and dried over night on air. A schematic view of the method is given in Figure 2. A first experiment with dehydrated zeolite 13× (dehydration by heating at 400˚C for 2 h) was performed to investigate the role of the hydrate water of the educt zeolite 13× as well as the significance of addition of solid NaOH for the whole reaction process. A second synthesis without NaOH but hydrated 13× was carried out too. The experimental data are summarized in Table 2.
-Analytical methods
X-ray powder diffraction (XRD), Fourier transform infrared spectroscopy

Figure 2. Schematic view of the experimental procedure of crossover synthesis from gel to melt.
Table 1. Experimental conditions and products of the autothermal synthesis series (A).
1)reaction period always 4 h; 2)( ): very few amount.
Table 2. Experimental conditions and products “SM1 - SM5” of synthesis from aqueous gel solution (S) to melt flow (M).
1): addition of 200 mg NaBH4; 2)holding time 1 h; 3)( ): very small amounts.
(FTIR), scanning electron microscopy (SEM) and thermogravimetry were used for analytical characterization of all products. The conversion of the reactants of the autothermal reactions was further controlled by weighting of the products on a Kern EMB 200-3 laboratory balance.
X-ray powder diffraction was performed on a a Philips PW-1800 diffracto- meter with Bragg-Brentano geometry and CuKα radiation in the 2 Theta range 5˚ - 85˚ (step width 0.03˚ and 1 sec measurement time per step, data evaluation with the WinXPow software (STOE)). Powder patterns of selected samples were analyzed by Rietveld refinements using the TOPAS 4.2 software (Bruker AXS). These patterns were measured in the 2 Theta range 2˚ - 80˚ at 0.02 s step width and 5 sec measurement time per step.
MIR-FTIR spectroscopy was carried out with a Bruker Vertex 80 v spectro- meter in the range of 400 to 4000 cm−1 (KBr wafer method: 1 - 2 mg sample/200 mg KBr).
Scanning electron microscopic (SEM) investigations were performed using a JEOL JSM-6390A SEM at an acceleration voltage of 30 kV.
The best products of each series were heated on a Setaram Setsys evolution 1750 thermoanalyzer up to 550˚C at a heating rate of 5˚C/min under atmosphere of synthetic air (80 Vol% N2, 20 Vol% O2; flow rate 20 ml/min). The thermal products were analyzed by XRD and FTIR to get information on hydrogen release and stabilities of the samples.
3. Results
-autothermal synthesis of NaBH4-sodalite
The results of phase analysis according XRD and quantitative evaluation of the products (amounts in g) are summarized in Table 1. The table also includes main experimental parameters of the autothermal syntheses.
The X-ray powder patterns of NaBH4 sodalites from autothermal syntheses are given in Figure 3. From this figure as well as Table 1 it can be derived that the product A 1 obtained in 4 M NaOH remains amorphous. The powder

Figure 3. X-ray powder patterns of products of the autothermal syntheses in the NaBH4- system.
pattern only consists of a broad signal in the 20˚ - 40˚ 2 Theta region caused by amorphous aluminosilicate beside a very strong background contribution. In contrast tetrahydroborate sodalite in adequate quality is already found under the conditions of the 8M NaOH solution in sample A2. Amounts of 70% crystalline tetrahydroborate sodalite beside 30% amorphous parts were calculated accord- ing Rietveld analysis.
Only small amounts of the amorphous phase were observed in the product A3 of 12 M NaOH synthesis whereas the fully crystalline product was obtained in 16 M NaOH. Here a lattice parameter a0 = 8.9336(4)Å was refined for sample A4. This cell parameter is somewhat larger, compared with the value a0 = 8.9161(2)Å of NaBH4 sodalite microcrystals from common hydrothermal synthesis. The cell parameter of sodalite reacts very sensitive on the species like (OH∙H2O)−, H2O or the [BH4]− anions enclathrated within the framework cavities. About 50% cage fillings with water as in expanded hydrosodalite [3] [6] can be derived from the refined cell parameter and a composition Na7[AlSiO4]6BH4(H2O)2 results for product A4 in accordance with FTIR and TG after subtraction of slightly adsorbed surface water leaving the sample in the 20˚C - 100˚C temperature interval (TG see below). The water molecules were incorporated during washing the sample by an exchange with (NaOH.H2O) cage fillings of the as synthesized product.
The FTIR spectra of autothermal synthesized NaBH4 sodalites are given in Figure 4. Whereas the spectrum of the product A1 from 4 M NaOH synthesis is typical for high amounts of amorphous contributions in the starting phase of crystallization, the 8M product A2 already shows much better resolved signals in the whole mid-infrared range. At last the spectra of the 12 - 16 M products are very well developed. A comparison of these three spectra (A2 - A4) shows close

Figure 4. FTIR spectra of products of the autothermal syntheses in the NaBH4-system.
similarities: the sodalite framework vibrations were clearly resolved in accor- dance with literature data [17] (bending modes at 450 cm−1 and 500 cm−1; triplet of the symmetric T-O-T vibrations [T = Si, Al] in the 660 cm−1 - 740 cm−1 range as well as the broad intense asymmetric T-O-T mode at around 1000 cm−1). A weak band at 650 cm−1 in the spectrum of the sample from synthesis in 8 M NaOH is expected to be caused by Al-O-Si vibrations of amorphous parts as known from aluminosilicate geopolymers [18] . The vibrations of the BH4− anions at 1143 (n4), 2286 (2*n4), 2241 (n3) and 2390 (n2 + n4) can be clearly observed in accordance with literature data [19] [20] [21] [22] . The bands are well resolved in the 12 - 16 M NaOH synthesis products and somewhat weaker in the 8M sample too.
Beside the BH4 anions even water within the samples can be detected according to typical bands in the FTIR spectra. The water molecules inside the sodalite cages of products A2, A3 and A4 and within the amorphous parts of sample A2 are responsible for the bands at 1650 cm−1 and 3100 cm−1 - 3700 cm−1 [23] . Beside cage water also slightly adsorbed “external surface water” has taken into account as the products were only dried on air before analysis to prevent any influence of higher temperatures during a drying procedure in a cabinet dryer. Impurities like some carbonate (from the mother liquor) were also detected due to the vibrations in the 1410 cm−1 - 1450 cm−1 region of the FTIR spectrum [23] , especially in sample A2.
SEM images of the products of autothermal NaBH4 sodalite synthesis in 8 M, 12 M and 16 M NaOH are summarized in Figure 5 (the amorphous sample of the synthesis in 4 M NaOH was excluded for SEM investigation). An image of a sample “BH4-hydro” observed under common hydrothermal conditions (20 ml

Figure 5. SEM photographs of products A2 - A4 of autothermal syntheses (Table 1) and a sample from conventional hydrothermal synthesis [2] “BH4-hydro” (down right).
16 M NaOH, temperature 120˚C, reaction time 24 h, as described in [2] ) is included in Figure 5 for comparison.
Small sodalite crystals of a size between 0.5 μm - 1.0 μm were observed in the product of autothermal synthesis with 8 M NaOH (Figure 5, top left), i.e. its crystal size is in the range as in the hydrothermal sample BH4-hydro (Figure 5, down right). In contrast to the more regular rhomben-dodecahedral habit of this hydrothermal sample the autothermal product consists of spherulitic crystals.
The product, obtained in 12 M NaOH consists of larger ball-like crystals with 2 - 4 μm diameters and a slightly rough surface. A few of these crystals are agglomerated to bigger aggregates (Figure 5, top right).
The NaBH4 sodalites synthesized with 16 M NaOH again look somewhat different. Spherulitic crystals of 1 - 2 μm now exhibit an edged surface roughness. Crystals appear as ball-like aggregates formed by intergrowths of thin lamellar shaped microcrystals. Big aggregates of agglomerated crystals with the same morphology were also observed within this batch (Figure 5, down left).
-NaBH4-sodalites by crossover synthesis from aqueous gel solution to NaOH melt flow
The results of phase analysis according XRD are summarized in Table 2. The table also includes main experimental parameters of crossover synthesis from gel to melt flow.
The X-ray powder patterns are summarized in Figure 6. Syntheses SM 1 and SM 2 were performed to clarify the combined reaction mechanism. Product SM 1, obtained with dehydrated zeolite 13-X clearly shows strong peaks of unconverted zeolite 13-X beside the main reflections of poor crystalline sodalite. Large amounts of amorphous material can be further seen according to the background contribution. Even synthesis SM 2 without addition of NaOH granulate consists only of zeolite 13-X and amorphous parts. These results are

Figure 6. X-ray powder patterns of products SM1-5 of synthesis from aqueous gel solution (S) to melt flow (M).
essential hints on the significance of water and NaOH for the successful run of the crossover synthesis from gel to melt.
All further experiments (SM 3 - 5) were performed with hydrated 13-X and NaOH granulate within the educt pellets (see experimental). The powder patterns of these three products all clearly indicate formation of sodalites, in product SM 3 together with 11% of amorphous material but in SM 4 and SM 5 as single phases. The lattice constants, added in Table 2 are in the range of the products from autothermal synthesis and again compositions up to 50% cage fillings with water according to Na7[AlSiO4]6(BH4)(H2O)2 can be derived. Anhydrous products were observed at first due to the high temperature of the melt step of crossover synthesis. But because the “as synthesized” samples contain about 50% cage fillings with hydroxide, again water incorporation results from exchange reaction during the washing process of the samples as known from the behavior of the series basic sodalite-hydrosodalite [24] [25] [26] .
The FTIR spectra of the products are summarized in Figure 7. Samples SM 1 and SM 2 both exhibit the bands of zeolite 13-X [17] . The vibration modes of the small amount of sodalite, formed within SM 1 are superimposed with the strong vibrations of the FAU-type framework of zeolite 13-X. Only very weak bands in the range of the tetrahydroborate vibrations (2286 cm−1 - 2390 cm−1) [19] [20] [21] [22] indicate traces of 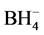 enclathrated sodalite within this product.
enclathrated sodalite within this product.
Exclusively well resolved typical vibrations of the sodalite framework can be seen in all the three spectra of samples SM 3 - 5: the strong broad band of asym- metric T-O-T stretching vibrations (T = Si, Al) with intensity maximum at ≈1000 cm−1, the symmetric T-O-T vibration modes (triplet in the 660 cm?1 - 740 cm?1 region) and the two strong bending modes at around 460 cm−1 and 430 cm−1―all in accordance with literature [17] .

Figure 7. FTIR spectra of products “SM1-5” of synthesis from aqueous gel solution (S) to Melt flow (M) and the spectrum of zeolite 13× for comparison.
Again the BH4 anions inside the toc units can be clearly distinguished by their characteristic vibration bands in the FTIR spectra too [19] [20] [21] [22] .
The spectra of sodalites SM 3 - 5 include further bands at 1650 cm−1 and 3100 cm−1 - 3700 cm−1, caused by water molecules within the products [23] . Beside this “internal cage water” slightly adsorbed “external surface water” has to be pointed out. The unusual loose-fitting agglomeration of nanocrystalls in form of large cubes, detected during SEM analysis (see below SEM image SM 4 in Figure 8) is responsible for many fine macroscopic cavities and channels between the agglomerated nanocrystals. Inclusion of some external surface water within those cavities and channels cannot be excluded.
SEM images of the products are summarized in Figure 8. In the product SM 3 nanocrystalline sodalites with average crystal dimensions around 100 nm were mainly observed beside some bigger particles around 1 µm size. The latter were formed by agglomeration of nanoparticles. It is assumed, that the nanoparticles are agglomerated by the amorphous parts, detected within this product.
Sample SM 4 contains big polyhedral aggregates, sometimes as sperulites some other like cubes of dimensions around 2 - 3 µm, but each aggregate again consists of a loosely aggregation of nanoparticles or tin platelet-like particles. The pores, cavities and channels formed thereby are favored for adsorption of “external water” from moisture when the sample is kept under open conditions.
In contrast sample SM 5 exhibits more spherulite-like crystals with an average size around 2 - 3 µm. Here again nanocrystals in form of very tin platelets are grown together to these denser particles as can be clearly seen at high magnifica-

Figure 8. SEM photographs of products SM3-5 of synthesis from aqueous gel solution (S) to melt flow (M); an image at higher magnification is inserted for sample SM 5 for better view of the surface roughness. For further comparison see also the sample from conventional hydrothermal synthesis already shown in Figure 5 (“BH4-hydro”; bottom right).
tion (image down left in Figure 8). Thus the nanocrystalline state of the samples and an agglomeration to bigger particles is observed for all products by SEM analysis. Again no crystals with the typical rhomben-dodecahedral habit of sodalites from common hydrothermal synthesis are found in any product of the crossover synthesis, just as in the products of the autothermal process (for comparison see the image of a products from common hydrothermal synthesis “BH4-hydro” down right in Figure 5).
-Hydrogen release properties of selected products of both synthesis procedures
The products A4 and SM 4 of both series were now further investigated by thermal analysis to observe the stability and reactivity of the enclathrated BH4 under the conditions of heating up to 550˚C in synthetic air atmosphere. Both samples were selected as best single phase and pure crystalline products nearly without amorphous byproducts, obtained in both different synthesis procedures. Regarding thermal reactions it is known from literature, that hydrogen release is possible by hydrolysis (starting at about 250˚C) or by oxidation reaction at elevated temperature according to Equations (4) and (5) [6] .
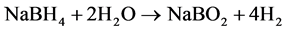 (4)
(4)
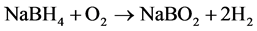 (5)
(5)
As reaction (5) requires high temperatures T > 400˚C [6] this oxidation process can be accompanied by the destruction of the sodalite framework at elevated temperature [6] . To investigate the hydrogen release properties of the synthesis products and the thermal stability of the host framework, both products (A4 and SM4) were heated on a thermoanalyzer up to 550˚C in synthetic air atmosphere (see experimental). The TG curves are given in Figure 9. X-ray powder diagrams of the samples after thermal analysis are summarized in Figure 10. Figure 11 gives an overview on the FTIR spectra of samples after thermal analysis in comparison with the as synthesized samples. Further data are summarized in Table 3.
The mass loss according to TG (Figure 9) indicates nearly the same water content within both samples (internal cage water plus external surface water) but it has to be taken into account that the overwhelming amount of water leaves the sample SM4 already at temperatures in the 200˚C - 250˚C range. In contrast, the
Table 3. Products, mass loss and dehydration characteristics after heating RT → 550˚C → RT in synthetic air atmosphere.


Figure 9. TG-curves for samples A4 and SM 4 during heating RT → 550˚C → RT in synthetic air atmosphere (heating rate 5˚C/min).

Figure 10. X-ray powder patterns of the selected samples A 4 and SM 4 after TG-analysis up to 550˚C (main reflections of carnegieite are labeled by stars).

Figure 11. FTIR spectra of the selected samples A 4 and SM 4 after TG-analysis up to 550˚C.
dehydration characteristics of sample A4 differs as remarkable amounts of water were released at higher temperatures. Thus a larger amount of water is therefore available for hydrolysis of BH4 in sample A4 at these temperatures.
From Figure 10 it can be seen, that the decomposition of the sodalite framework already starts for both samples at 550˚C, as the main reflections of the secondary product low-carnegieite (main lines labeled by stars) are found in the XRD patterns of A4 and SM 4 after thermal process.
The FTIR investigation (Figure 11) clearly shows that the overwhelming amount of hydrogen has left the sodalite cages of sample A4 as strong signals of metaborate BO2 and BO(OH)2 anions [27] are well resolved in the FTIR spectrum. This behavior indicates hydrolysis reaction under conversion of the “internal water” enlarged in the cages of “hydrosodalite-type fillings” of sample A4 as the dominant reaction mechanism. In contrast, the product from reaction of gel-like solution and melt flow (SM 4) exhibits a lower hydrogen release rate that is not completed during heating up to 550˚C. The vibrations of the BH4-groups remain in remarkable intensity beside the bands of the borate species BO2 and BO(OH)2 (see spectrum “SM4 550˚C to RT”, Figure 11). As the product SM4 exhibits similar water contents as A4 but shows lower temperature of dehydration as result of its nano-sized crystals hydrolysis reaction seems to be restricted and oxidation reaction will be the process for further hydrogen release. The dehydration characteristics of SM4 further show that the water molecules inside the cages and the external surface water already leave at remarkable lower temperature. At 250˚C (i.e. the temperature where hydrolysis reaction usually starts [6] ) the sample is already widely dehydrated. Hydrogen release by hydrolysis is thus restricted and oxidation reaction would be the mechanism for further release of H2. Additional investigations are necessary to clarify this behavior completely.
Figure 12 includes a SEM photograph of samples A4 and SM 4 after the heat-

Figure 12. SEM images of the selected samples A 4 and SM 4 after TG-analysis up to 550˚C.
ing procedure RT → 550˚C → RT in synthetic air atmosphere. It can be seen that the agglomerated crystals of sample A4 are now more separated into single microcrystals of spherical habit and about 1 µm diameter. In contrast the nanoparticles of sample SM 4 still remain in its agglomerated state.
4. Conclusions
NaBH4 sodalites can be obtained by two new modified methods of crystallization: (1) autothermal synthesis and (2) crossover synthesis from aqueous gel to melt flow in a NaOH flux. Important parameters like crystal size, crystal morphology, composition and hydrogen release mechanism (hydrolysis and oxidation) can be influenced in dependence of the inserted method of synthesis.
Beside tailoring of the products the enhanced methods of crystallization exhibit many other important peculiarities:
The generation of the whole process energy by the enthalpy of the exothermic reaction system without any external heating has to be mentioned here for procedure (1). Method (2) demonstrates the successful insertion of zeolite water of the hydrated educt zeolite 13× as the only water source for synthesis. Short reaction times and the abandonment of autoclaves are further advantages of both methods.
Many further possibilities are offered by the new synthesis pathways, even in other material systems than sodalites. Future optimization of conditions of both reactions is essential to obtain other special types of materials. As an example therefore maybe crystal coarsening and sinter processes at elevated Tmax. and/or prolonged heating times under conditions of method (2) surely yield to dense self-supported membrane-like wafers of NaBH4 sodalite.
Generalizing the results of the present study one can state that two modified experimental methods are presented with future significance for tailor made synthesis even under insertion of sensitive compounds like NaBH4.
Cite this paper
Buhl, J.-Ch. (2017) NaBH4 Sodalites, Synthesized by Modified Methods: (1) Autothermal Synthesis and (2) Crossover Reaction from Gel to Melt Flow. Advances in Chemical Engineering and Science, 7, 108-124. https://doi.org/10.4236/aces.2017.72009
References
- 1. Davis, R.E., Bromels, E. and Kibby, C.L. (1962) Boron Hydrides. III. Hydrolysis of Sodium Borohydride in Aqueous Solution. Journal of the American Chemical Society, 84, 885-892.
https://doi.org/10.1021/ja00865a001 - 2. Buhl, J.-C., Gesing, T.M. and Rüscher, C.H. (2005) Synthesis, Crystal Structure and Thermal Stability of Tetrahydroborate Sodalite Na8[AlSiO4]6(BH4)2. Microporous and Mesoporous Materials, 80, 57-63.
https://doi.org/10.1016/j.micromeso.2004.11.022 - 3. Buhl, J.-C., Schomborg, L. and Rüscher, C.H. (2010) Tetrahydroborate Sodalite Nanocrystals: Low Temperature Synthesis and Thermally Controlled Intra Cage Reactions for Hydrogen Release of Nano- and Micro Crystals. Microporous and Mesoporous Materials, 132, 210-218.
https://doi.org/10.1016/j.micromeso.2010.02.022 - 4. Buhl, J.-C., Gesing, T.M., Hofs, T. and Rüscher, C.H. (2006) Synthesis and Crystal Structure of Gallosilicate- and Aluminogermanate Tetrahydroborate Sodalites Na8[GaSiO4]6(BH4)2 and Na8[AlGeO4]6(BH4)2. Journal of Solid State Chemistry at Science, 179, 3877-3882.
https://doi.org/10.1016/j.jssc.2006.08.031 - 5. Höfs, T.K. and Buhl, J.-C. (2011) Thermal Behavior of NaBH4-Sodalites with Alumosilicate Framework: Influence of Cage Water Content and the Surrounding Conditions. Materials Research Bulletin, 46, 1173-2178.
- 6. Buhl, J.-C., Schomborg, L. and Rüscher, C.H. (2012): Enclosure of Sodium Tetrahydroborate (NaBH4) in Solidified Aluminosilicate Gels and Microporous Crystalline Solids for Fuel Processing. In: Liu, J., Ed., Hydrogen Storage, InTech, 49-90.
https://doi.org/10.5772/50186 - 7. Pauling, L. (1930) The Structure of Sodalite and Helvite. Zeitschrift für Kristallographie, 74, 213-225.
- 8. Löns, J. and Schulz, H. (1967) Strukturverfeinerung von Sodalith Na8Si6Al6O24Cl2. Acta Crystallographica, 23, 434-436.
https://doi.org/10.1107/S0365110X67002920 - 9. Baerlocher, C., Meier, W.M. and Olson, D.H. (2001) Atlas of Zeolite Framework Types. 5th Edition, Elsevier, Amsterdam.
- 10. Buhl, J.-C. (2016) On the Autothermal Synthesis of Zeolites. Microporous and Mesoporous Materials, 222, 73-79.
https://doi.org/10.1016/j.micromeso.2015.10.004 - 11. Buhl, J.-C. (2016) Enhanced Methods of Crystallization: The Crossover Synthesis from Aqueous Solution to Melt Flow. Zeitschrift für Kristallographie, 36, 5.
- 12. Petrovic, J. and Thomas, G. (2008) Reaction of Aluminum with Water to Produce Hydrogen. US Dept. of Energy, 1-26.
- 13. Belitskus, D. (1970) Reaction of Aluminum with Sodium Hydroxide Solution as a Source of Hydrogen. Journal of the Electrochemical Society, 117, 1097-1099.
https://doi.org/10.1149/1.2407730 - 14. Hadan, M. and Fischer, F. (1992) Synthesis of Fine Grained NaA-Type Zeolites from Superalkaline Solutions. Crystal Research and Technology, 27, 343-350.
https://doi.org/10.1002/crat.2170270310 - 15. Fischer, F., Hadan, M. and Horn, A. (1991) Investigations to the Synthesis of Zeolite Na A for Using in Detergents from Superalkaline Solutions. Chemische Technik, 43, 191-195.
- 16. Breck, D.W. (1974) Zeolite Molecular Sieves: Structure, Chemistry and Use. John Wiley & Sons Inc., New York.
- 17. Flanigen, E.M., Khatami, H. and Szymanski, H.A. (1971) Infrared Structural Studies of Zeolite Frameworks. Advances in Chemistry, 101, 201-209.
https://doi.org/10.1021/ba-1971-0101.ch016 - 18. Kriven, W.M., Bell, J.L., Gordon, M. and Wen, G. (2005) Geopolymers: More than Just Cements, Geopolymer, Green Chemistry and Sustainable Development Solutions. Geopolymer Institute, Quentin.
- 19. Waddington, T.C. (1958) Thallous Borohydride TlBH4. Journal of the Chemical Society, 4783-4784.
- 20. Goubeau, J. and Kallfass, H. (1959) Die Reaktion von Natriumborhydrid und Wasser. Zeitschrift für Anorganische und Allgemeine Chemie, 299, 160-169.
https://doi.org/10.1002/zaac.19592990308 - 21. Schutte, C.J.H. (1960) The Infrared Spectrum of Thin Films of Sodium Borohydride. Spectrochimica Acta, 16, 1054-1059.
https://doi.org/10.1016/0371-1951(60)80145-2 - 22. Ketelaar, J.A.A. and Schutte, C.J.H. (1961) The Boronhydride Ion (BH4-) in a Face Centered Cubic Alkali-Halide Salt. Spectrochimica Acta, 17, 1240-1243.
https://doi.org/10.1016/0371-1951(61)80046-5 - 23. Weidlein, J. and Müller, U. and Dehnicke, K. (1981) Schwingunsfrequenzen. Georg Thieme Vlg., Stuttgart.
- 24. Engelhardt, G., Felsche, J. and Sieger, P. (1992) The Hydrosodalite System Na6+x[AlSiO4]6(OH)x.n H2O: Formation, Phase Composition and de- and Rehydration Studied by 1H, 23Na and 29Si MAS-NMR Spectroscopy in Tandem with Thermal analysis, X-Ray Diffraction and IR Spectroscopy. Journal of the American Chemical Society, 114, 1173-1182.
https://doi.org/10.1021/ja00030a008 - 25. Felsche, J. and Luger, S. (1986) Structural Collapse or Expansion of the Hydrosodalite Series Na8[AlSiO4]6(OH)2.n H2O or Na6[AlSiO4]6.n H2O upon Dehydration. Berichte der Bunsengesellschaft für Physikalische Chemie, 90, 731-736.
https://doi.org/10.1002/bbpc.19860900822 - 26. Felsche, J. and Luger, S. (1987) Phases and Thermal-Decomposition Characteristics of Hydro-Sodalites Na8[AlSiO4]6(OH)x.nH2O. Thermochimica Acta, 118, 35-55.
https://doi.org/10.1016/0040-6031(87)80069-2 - 27. Pietsch, H.H.E., Fechtelkord, M. and Buhl, J.-C. (1997) The Formation of Unusual Twofold Coordinated Boron in a Sodalite Matrix. Journal of Alloys and Compounds, 257, 168-174.
https://doi.org/10.1016/S0925-8388(97)00019-4
上一篇:Solubility of Solids in Superc 下一篇:Hydrothermal Dissolution of Op
最新文章NEWS
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Solubility of Solids in Supercritical Fluids: The Mendez-Santiago Teja Model Revisited
- Solventless Organic Reactive Crystallization at Mild Conditions
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Luminescent Characteristic of Organic Compound-Containing Inorganic Crystal at Room Temperature
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Tunable Polymorphic Transformation Temperature
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- A Convective Thin Layer Drying Model with Shrinkage for Kent Mango Slices
- Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment
- Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries
- Optimisation of Decolourisation Conditions of Crude Shea (Vitellaria paradoxa Gaertner F) Butter: Ye
- Influence of Bed Geometry on the Drying of Skimmed Milk in a Spouted Bed
- Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp.
- Mechanism of the Products Formation in the Vapor Phase Ammoxidation Reaction of 4-Phenyl-o-Xylene
- Solvent Extraction of Citric Acid with Different Organic Phases

