Development of Aseptic Renal Abscess in a Patient with Non-Small-Cell Lung Cancer with ALK Transloca
Vol.04No.04(2015), Article ID:63949,5 pages
10.4236/alc.2015.44007
Luciana Franco do Prado de Carvalho1*, Andrea Kazumi Shimada1, Manuel Santos da Cruz Neto1, Lucila Soares da Silva Rocha1, Publio Cesar Cavalcante Viana2, Esper George Kallas3, Artur Katz1
1Centro de Oncologia, Hospital Sírio-Libanês, Sao Paulo, Brazil
2Radiologia Intervencionista, Hospital Sírio-Libanês, Sao Paulo, Brazil
3Infectious Disease, Hospital Sírio-Libanês, Sao Paulo, Brazil

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 January 2016; accepted 26 February 2016; published 29 February 2016
ABSTRACT
Background: Crizotinib is a tyrosine kinase inhibitor of ALK, MET and ROS1. In a safety database trial, it was suggested an association of Crizotinib with the development of renal cyst in patients with non-small-cell lung cancer (NSCLC). Aim: To report an uncommon side effect of Crizotinib in a patient with NSLC. Case Presentation: We report the case of a 68-year-old woman with NSCLC who developed bilateral progressive aseptic renal abscesses during Crizotinib treatment. Conclusion: Further studies may be necessary to determinate the risk of renal cyst development and the management of these complications.
Keywords:
Non-Small-Cell Lung Cancer, Crizotinib, Renal Abscesses

1. Introduction
The activating mutation or translocation of anaplastic lymphoma kinase gene (ALK) is present at 2% to 7% of non-small cell lung cancer (NSCLC) [1] [2] and it is also described on large cell linfoma [3] and neuroblastoma [4] . The prevalence of ALK mutation in NSCLC is higher in patients with adenocarcinoma who have little or no exposure to tobacco [2] [5] .
Crizotinib is a selective oral inhibitor of small tyrosine kinase molecules ALK, mesenchymal-epithelial transition factor (MET) and ROS1 that inhibits the phosphorylation and thus activation of the ALK, MET and ROS1 through competitive inhibition with ATP [2] [6] [7] .
Phase 3 trials showed that the inhibition of ALK with Crizotinib in lung tumors with the ALK rearrangement resulted in benefit in progression-free survival, symptom control and response rate in both first and second line with response rate of 68% - 75% and progression-free survival of 10.9 months [8] [9] .
Common adverse events associated with Crizotinib are nausea, vomiting, fatigue, visual change, change in appetite, edema lifting transaminases and interstitial lung disease [8] [10] .
PROFILE 1005 [11] , a phase II trial, reported the first two cases of renal cyst during Crizotinib treatment. Other cases were reported subsequently, suggesting a causal association with Crizotinib [11] .
In a retrospective analysis, among 1205 patients who received Crizotinib in the two largest Crizotinib clinical trials (PROFILE 1005 [11] and PROFILE 1007 [9] ), 24 patients (2%) had adverse effects of treatment-related renal cysts [12] . In four clinical trials, 17 patients had renal cysts reported as serious adverse event [12] .
In a blinded retrospective radiologic review of 272 patients treated with Crizotinib enrolled in PROFILE 1001 [10] , PROFILE 1005 [11] , or PROFILE 1007 [9] , in six months, 9% of the patients developed renal cysts. Seventeen patients had severe side effects related to complex renal cysts, such as invasion of adjacent structures [12] .
We report a case of a patient with NSCLC ALK translocation who developed multiple, bilateral aseptic renal abscesses on treatment with Crizotinib.
2. Case Report
We report a 68-year-old woman diagnosed with NSCLC with a recurrent, stage IV adenocarcinoma of the lung with ALK rearrangement who was treated in third line with Crizotinb. At the time of treatment initiation, the abdominal CAT scan showed only the presence of subcentrimetric renal cysts, which have been unchanged for several years. Crizotinib was started and shortly after a PET-CT documented tumor shrinkage.
Six months after the treatment was she developed intermittent dysuria and weight loss, while urine analysis remained normal. MRI of the abdomen showed new nodular lesions with intermediate signal and significant restriction of diffusion, in both kidneys. These lesions were not coincident with the previously known renal cysts (Figure 1).
An abdominal MRI was repeated 8 weeks later and showed these lesions as cystic/liquefied images with intermediate signal in T2 and significant restriction, increased in size and number. There was no evidence of tumor progression.
A percutaneous puncture and drainage of the cyst at the upper pole of the left kidney guided by computed tomography was performed, with the suspicion of infection. At the procedure, 6 ml of purulent fluid were removed (Figure 2). Microbiological, including direct evaluation for microorganisms, cultures and PCR analysis were all negative.
After a month, a repeated MRI showed that the patient had developed a new lesion on the left kidney (Figure 3(A) and Figure 3(B)). A second CT-guided procedure was undertaken, and the both the right and left sided lesions underwent drainage. Once again purulent liquid was removed from the lesions and a thorough microbiological analysis was ruled out an infection process.
An MRI performed 4 weeks after the second procedure showed that the lesions continued to grow in size and numbers. At the same time a PET-CT showed that the disease remained in partial remission. At this point, Crizotinib was discontinued and Ceritinib was started (Figure 3(C) and Figure 3(D)). The following MRI showed progressive resolution of the renal lesions.
The patient is alive, using Ceritinib for 10 months with stable disease and resolution of the renal lesions.
The mechanism on the development of Crizotinib-associated complex renal cysts is unclear [5] and apparently it not shared with Ceritinib, given the resolution of the lesions. Physicians need to be aware of this potential uncommon side effect of this drug, given that its use will likely increase worldwide in the coming years, as the drug becomes available in multiple countries and as adjuvant trials are currently recruiting patients.

Figure 1. Right kidney (A) and (B) and left kidney (C) and (D) before Crizotinibe and after treatment (E), (F), (G), (H).

Figure 2. A percutaneous puncture and drainage of the cyst at the upper pole of the left kidney guided by CT.
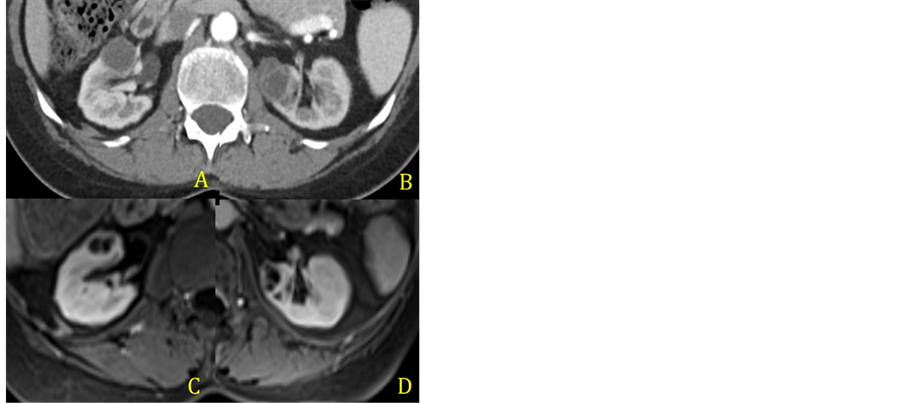
Figure 3. CT scan after percutaneous puncture and drainage of the renal abcess (A) and (B) and MRI after Crizotinib was discontinued (C) and (D).
3. Discussion
The incidence of renal cysts occurring during treatment with Crizotinib is about 5% [13] . Crizotinib increases the risk of renal cysts development and also increases pre-existing cystic lesions [12] [14] .
Six months after Crizotinib, our patient presented with new symptoms of urinary infection and the CT scans already showed nodular lesions with intermediate signal and significant restriction of diffusion in both kidneys . In the retrospective analysis by Schell et al. of the 17 patients who developed renal cysts reported in clinical trial as major adverse events, the median time to diagnosis of renal cysts was 6.6 months (from 1.2 to 15.2 months) after start of treatment. In his analysis, 3 patients had to have dose reduced, the others were kept on regular doses without harm. Only one patient had to suspend treatment due to disease progression [12] . Other case report of a patient in Japan also showed spontaneous regression of a complex renal cyst [15] .
In our case, the patient presented increase of the abscess content after the reintroduction of Crizotinib, so we opt for the suspension of Crizotinib with significant decrease of the lesions. Lin et al. [14] evaluated 32 patients in 3 clinical trial and also found significant reduction in the size of renal cysts after treatment suspension. The patients who had significant renal cyst changes received Crizotinib for longer duration (median, 956 days versus 248 days, p = 0.007). In our case, the patient developed symptoms after just 6 months of the treatment, with significant changes in CT scans. After suspension, patient had evolved with clinical e radiological control without further intervention.
Schnell et al. [12] described only one patient of 17 with renal abscess [12] . They did not report any pathologic agent in this case. Yoneshima et al. [16] also described a case of a patient with aseptic renal abscess after treatment with Crizotinib in Japan.
We changed the patient treatment for Ceretinib, a drug that is also a selective oral inhibitor of small tyrosine kinase molecules that target ALK and ROS1 but it seems to have no relation to the formation of renal cysts. Despite have the same target, that is no data regarding Ceretinib and renal cyst development [17] . Our patient evolved with clinical and radiological improvement after the discontinuation of the drug.
4. Conclusion
We describe a case of a patient with NSCLC with ALK rearrangement, who presented with sub-centimeter renal cysts before Crizotinib treatment and developed bilateral renal abscess after six months of initiating the drug. The mechanism of Crizotinib related renal cyst is unknown but does not seem to have relation with the inhibition of ALK or ROS1. Further studies may be necessary to determinate the risk of renal cyst development and management of the drug’s complications.
Cite this paper
Luciana Franco do Pradode Carvalho,Andrea KazumiShimada,Manuel Santosda Cruz Neto,Lucila Soaresda Silva Rocha,Publio Cesar CavalcanteViana,Esper GeorgeKallas,ArturKatz, (2015) Development of Aseptic Renal Abscess in a Patient with Non-Small-Cell Lung Cancer with ALK Translocation during Crizotinib Treatment. Advances in Lung Cancer,04,53-57. doi: 10.4236/alc.2015.44007
References
- 1. Soda, M., Choi, Y.L., Enomoto, M., et al. (2007) Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature, 448, 561-566.
http://dx.doi.org/10.1038/nature05945 - 2. Kwak, E.L., Bang, Y.J., Camidge, D.R., et al. (2010) Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. New England Journal of Medicine, 363, 1693-1703.
http://dx.doi.org/10.1056/NEJMoa1006448 - 3. Kutok, J.L. and Aster, J.C. (2002) Molecular Biology of Anaplastic Lymphoma Kinase-Positive Anaplastic Large-Cell Lymphoma. Journal of Clinical Oncology, 20, 3691-3702.
http://dx.doi.org/10.1056/NEJMoa1006448 - 4. George, R.E., Sanda, T., Hanna, M., et al. (2008) Activating Mutations in ALK Provide a Therapeutic Target in Neuroblastoma. Nature, 455, 975-978.
http://dx.doi.org/10.1038/nature07397 - 5. Wong, D.W., Leung, E.L., So, K.K., et al. (2009) The EML4-ALK Fusion Gene Is Involved in Various Histologic Types of Lung Cancers from Nonsmokers with Wild-Type EGFR and KRAS. Cancer, 115, 1723-1733.
http://dx.doi.org/10.1002/cncr.24181 - 6. Ou, S.H., Kwak, E.L., Siwak-Tapp, C., et al. (2011) Activity of Crizotinib (PF02341066), a Dual Mesenchymal-Epithelial Transition (MET) and Anaplastic Lymphoma Kinase (ALK) Inhibitor, in a Non-Small Cell Lung Cancer Patient with de novo MET Amplification. Journal of Thoracic Oncology, 6, 942-946.
http://dx.doi.org/10.1097/JTO.0b013e31821528d3 - 7. Bergethon, K., Shaw, A.T., Ou, S.H., et al. (2012) ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. Journal of Clinical Oncology, 30, 863-870.
http://dx.doi.org/10.1200/JCO.2011.35.6345 - 8. Solomon, B.J., Mok, T., Kim, D.W., et al. (2014) First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. New England Journal of Medicine, 371, 2167-2177.
http://dx.doi.org/10.1056/NEJMoa1408440 - 9. Shaw, A.T., Kim, D.W., Nakagawa, K., et al. (2013) Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. New England Journal of Medicine, 368, 2385-2394.
http://dx.doi.org/10.1056/NEJMoa1214886 - 10. Camidge, D.R., Bang, Y.J., Kwak, E.L., et al. (2012) Activity and Safety of Crizotinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer: Updated Results from a Phase 1 Study. Lancet Oncology, 13, 1011-1019.
http://dx.doi.org/10.1016/s1470-2045(12)70344-3 - 11. Kim, D.W., Ahn, M.J., Shi, Y., et al. (2012) Results of a Global Phase II Study with Crizotinib in Advanced ALK-Positive Non-Small Cell Lung Cancer (NSCLC). Journal of Clinical Oncology, 30, Abstract 7533.
- 12. Schnell, P., Bartlett, C.H., Solomon, B.J., Tassell, V., Shaw, A.T., de Pas, T., et al. (2015) Complex Renal Cysts Associated with Crizotinib Treatment. Cancer Medicine, 4, 887-896.
http://dx.doi.org/10.1002/cam4.437 - 13. XALKORI U.S. Physician Prescribing Information. Accessed 15 May 2014.
http://labeling.pfizer.com/ShowLabeling.aspx?id=676 - 14. Lin, Y.-T., Wang, Y.-F., Yang, J.C., Yu, C.-J., Wu, S.-G., Shih, J.-Y. and Yang, P.-C. (2014) Development of Renal Cysts after Crizotinib Treatment in Advanced ALK-Positive Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology, 9, 1720-1725.
http://dx.doi.org/10.1097/JTO.0000000000000326 - 15. Klempner, S.J., Aubin, G., Dash, A. and Ou, S.H. (2014) Spontaneous Regression of Crizotinib-Associated Complex Renal Cysts during Continuous Crizotinib Treatment. Oncologist, 19, 1008-1010.
http://dx.doi.org/10.1634/theoncologist.2014-0216 - 16. Yoneshima, Y., Okamoto, I., Arimura-Omori, M., Kimura, S., Hidaka-Fujimoto, N., Iwama, E., Harada, T., Takayama, K. and Nakanishi, Y. (2015) Infected Complex Renal Cysts during Crizotinib Therapy in a Patient with Non-Small Cell Lung Cancer Positive for ALK Rearrangement. Investigational New Drugs, 33, 510-512.
http://dx.doi.org/10.1007/s10637-014-0195-1 - 17. ZYCARDIA (2016) Physician Prescribing Information.
http://hcp.novartis.com/products/zykadia/alk-nsclc/safety-profile/
NOTES

*Corresponding author.
上一篇:Circulating IgG antibody again 下一篇:PGRMC1 Elevation in Multiple C
最新文章NEWS
- Missing the Target?—Targeted Therapy in Small Cell Lung Cancer
- Prognostic Role of miR-205 in Early-Stage (T1N0) Non-Small Cell Lung Cancer
- Radiation-Induced Lung Cancers in Murine Models
- Gingival Metastasis Revealing Lung Adenocarcinoma
- Usage of Cox-Regression Model for Forecasting of Survival Rate in Patients with the Early Sta
- Five-Year Survivors of Non-Small Cell Lung Cancer Patients with Positive Pleural Lavage Cytology
- A Phase II Study of Erlotinib in Patients with Previously Treated Non-Small Cell Lung Cancer
- Feasibility Study for Biweekly Administration of Cisplatin plus Vinorelbine as Adjuvant-Chemotherap
推荐期刊Tui Jian
- Chinese Journal of Integrative Medicine
- Journal of Genetics and Genomics
- Journal of Bionic Engineering
- Pedosphere
- Chinese Journal of Structural Chemistry
- Nuclear Science and Techniques
- 《传媒》
- 《中学生报》教研周刊
热点文章HOT
- Prognostic Significance of Pigment Epithelium-Derived Factor Expression in Patients with Non-Small-C
- Usage of Cox-Regression Model for Forecasting of Survival Rate in Patients with the Early Sta
- Diagnostic and Prognostic Value of Survivin in Pleural Effusion
- Differentiation of Benign and Malignant Solitary Pulmonary Nodule: Literature Review
- Development of Aseptic Renal Abscess in a Patient with Non-Small-Cell Lung Cancer with ALK Transloca
- Rate of Recurrence of Non-Small Cell Lung Cancer in Patients Treated with Percutaneous Ablation
- Gingival Metastasis Revealing Lung Adenocarcinoma
- Feasibility Study for Biweekly Administration of Cisplatin plus Vinorelbine as Adjuvant-Chemotherap
