Effects of Calcium on the GABA<sub>A</sub>-Coupled CI<sup>-</sup>/HCO<sub
Advances in Enzyme Research
Vol.2No.2(2014), Article ID:46656,10 pages DOI:10.4236/aer.2014.22009
Sergey A. Menzikov*, Marina V. Kalinina
State Research Institute of General Pathology and Pathological Physiology RAS, Moscow, Russia
Email: *menzikov@mail.ru, *sambrainic@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 April 2014; revised 10 May 2014; accepted 21 May 2014
The work is a study of the influence of Ca2+ (0.01 - 1 mM) on neuronal Cl−, 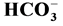 -ATPase complex: an enzyme that is a Cl−-pump which is functionally and structurally coupled to GABAA-receptors. It is found that influence of Ca2+ on the multifunctional complex starts at concentration of 50 (M and at concentration of 0.1 mM, it reduces the “basal” one and increases the Cl−,
-ATPase complex: an enzyme that is a Cl−-pump which is functionally and structurally coupled to GABAA-receptors. It is found that influence of Ca2+ on the multifunctional complex starts at concentration of 50 (M and at concentration of 0.1 mM, it reduces the “basal” one and increases the Cl−, 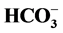 -stimulated Mg2+-ATPase activities. GABA (0.1 - 100 (M) activates the “basal” Mg2+-ATPase activity in the absence of calcium. The effect of GABA on the enzyme in the presence of 0.01 (M Ca2+ does not change. At the same time, 1 mM Ca2+ eliminates the GABA effect on the “basal” Mg2+-ATPase activity. Competitive blocker of GABAA-receptors bicuculline (5 - 20 (M) in the absence of Ca2+ ions eliminates the stimulation of the “basal” Mg2+-ATPase by anions. When 0.25 mM Ca2+ is added to the incubation medium the inhibitory bicuculline effect on the enzyme does not appear. We found that 0.1 mM o-vanadate (protein tyrosine phosphatase blocker) reduces the GABA-activated ATPase activity. At the same time, 0.1 mM genistein (a protein tyrosine kinase blocker) has no effect on enzyme activity. In the presence of Ca2+ (0.25 mM), the effect of o-vanadate on the “basal” and Cl−,
-stimulated Mg2+-ATPase activities. GABA (0.1 - 100 (M) activates the “basal” Mg2+-ATPase activity in the absence of calcium. The effect of GABA on the enzyme in the presence of 0.01 (M Ca2+ does not change. At the same time, 1 mM Ca2+ eliminates the GABA effect on the “basal” Mg2+-ATPase activity. Competitive blocker of GABAA-receptors bicuculline (5 - 20 (M) in the absence of Ca2+ ions eliminates the stimulation of the “basal” Mg2+-ATPase by anions. When 0.25 mM Ca2+ is added to the incubation medium the inhibitory bicuculline effect on the enzyme does not appear. We found that 0.1 mM o-vanadate (protein tyrosine phosphatase blocker) reduces the GABA-activated ATPase activity. At the same time, 0.1 mM genistein (a protein tyrosine kinase blocker) has no effect on enzyme activity. In the presence of Ca2+ (0.25 mM), the effect of o-vanadate on the “basal” and Cl−, 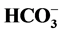 -ATPase activities does not appear. It is shown for the first time that high concentrations of Ca2+ prevent the action of GABAA-ergic ligands on the study ATPase. It is assumed that there is the involvement of protein kinases and protein phosphatases in the modulation of the enzyme activity by calcium. The observed effect of calcium on the ATPase may play an important role in the study of the mechanisms of epileptogenesis and seizure activity.
-ATPase activities does not appear. It is shown for the first time that high concentrations of Ca2+ prevent the action of GABAA-ergic ligands on the study ATPase. It is assumed that there is the involvement of protein kinases and protein phosphatases in the modulation of the enzyme activity by calcium. The observed effect of calcium on the ATPase may play an important role in the study of the mechanisms of epileptogenesis and seizure activity.
Keywords:Mg2+-ATPase, Chloride, Bicarbonate, Calcium, Rat Brain Plasma Membranes, GABAA-Ergic Drugs, o-Vanadate, Genistein

Cl−-ATPase/Cl−-pump in plasma membrane from various cells (including neurons) is a “molecular machine” participating in the transportation of 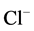 ions against the electrochemical gradient [1] [2] . Earlier, we showed the existence of the anion-sensitive Mg2+-ATPase in developed neurons of animal brain with maximum activity in the presence of Cl−/
ions against the electrochemical gradient [1] [2] . Earlier, we showed the existence of the anion-sensitive Mg2+-ATPase in developed neurons of animal brain with maximum activity in the presence of Cl−/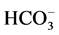 ions (in the ratio of as 5:1) [3] . These data are in line with the electrophysiological studies demonstrating that
ions (in the ratio of as 5:1) [3] . These data are in line with the electrophysiological studies demonstrating that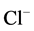 ,
, 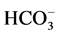 anions are transported through the GABAА-receptor Cl−-channel in the ratio as 5:1, respectively [4] . Besides, this enzyme is functionally and structurally coupled with the GABAА/ benzodiazepine receptor complex [3] . With the use of biochemical and cytochemical methods, it has been established that
anions are transported through the GABAА-receptor Cl−-channel in the ratio as 5:1, respectively [4] . Besides, this enzyme is functionally and structurally coupled with the GABAА/ benzodiazepine receptor complex [3] . With the use of biochemical and cytochemical methods, it has been established that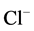 ,
, 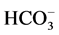 -ATPase is localized in elements of GABAА-ergic dendro-dendritic synapses [5] . The hydrolytic activity of this GABAА-coupled ATPase provides the
-ATPase is localized in elements of GABAА-ergic dendro-dendritic synapses [5] . The hydrolytic activity of this GABAА-coupled ATPase provides the 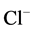 /
/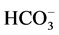 -transport processes with energy and determines the direction of ion transport through neuronal membrane [6] . This conclusion is based on the following findings: the protein preferably hydrolyzes ATP and is covalently phosphorylated by ATP (directly or with the participation of protein kinase) during the transport cycle and is dephosphorylated by anions [7] . These data allow us to suppose that this is a chloride transporting ATPase: this multifunctional ATPase is a
-transport processes with energy and determines the direction of ion transport through neuronal membrane [6] . This conclusion is based on the following findings: the protein preferably hydrolyzes ATP and is covalently phosphorylated by ATP (directly or with the participation of protein kinase) during the transport cycle and is dephosphorylated by anions [7] . These data allow us to suppose that this is a chloride transporting ATPase: this multifunctional ATPase is a  -pump participating in transporting chloride ions through the native and artificial membranes of liposomes.
-pump participating in transporting chloride ions through the native and artificial membranes of liposomes.
Furthermore, we have found that such ATPase is involved in rat convulsant-induced seizure activity [8] . It is known that the pathogeneses of epilepsy and convulsive states are associated not only with impairment of the GABAA-receptor function [9] [10] but also with calcium homeostasis [11] . It is known that the level of intracellular free calcium [Ca2+]i in the nerve cells is vital for the process of neuronal transmission [12] . Moreover, cations of calcium and magnesium are required to maintain the functional activity of many receptors and enzyme systems (including the GABAA-receptor) [13] . The influence of [Ca2+]i on the GABAA-receptors in the brain is exerted either directly through binding sites on the receptor molecule [14] or via Ca2+-dependent enzymatic processes (including protein kinases or protein phosphatases) [15] . In this regard, in the present work we have studied the influence of Ca2+ on the multifunctional ATPase activity of the neuronal membranes in the absence and in the presence of GABAA-ergic ligands and blockers of protein kinases and protein phosphatases.
2.1. Animals
Experiments were performed on male Wistar rats weighing 180 - 200 g. Animals were maintained under standard vivarium conditions with free access to water and food. The experiment was conducted under the “Rules of work with experimental animals” FGBU “NIIOPP” RAS, which comply with the World Society for the Protection of Animals (WSPA) and the European Convention for the protection of experimental animals.
2.2. Isolation of Plasma Membrane
All procedures were performed at 0˚C - 4˚C. After decapitation of animals, the brain was isolated, homogenized in 8 vol. of ice-cold buffer solution containing 0.25 M sucrose, 1 mM ethylenediaminetetraacetic acid-Tris (hydroxymethyl) aminomethane (EDTA-Tris, pH 7.4), 12.5 mM N-(2-Hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid) (HEPES-Tris, pH 7.4), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 50 units/ml aprotinin and centrifuged in a Beckman ultracentrifuge (SW-28 bucket rotor) at 10,000 × g and 4˚C for 25 min. The supernatant was centrifuged at 100,000 × g and 4˚C for 1 h. The supernatant was discarded and microsomal fraction enriched plasma membranes (pellet) was resuspended in 1 mM EDTA-Tris (pH 7.4), 12.5 mM HEPES-Tris (pH 7.4), stirred for 15 min and centrifuged (100,000 × g, 45 min). The resulting pellets were resuspended in 12.5 mM HEPES-Tris (pH 7.4) and frozen at −80˚C. This plasma membrane rich fraction was used for further measurements of the ATPase activity.
2.3. Assay of ,
,  -ATPase Activity
-ATPase Activity
The enzyme preparation (20 - 25 μg) was added to 0.5 ml incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM ATP-Tris, 10 mM NaCl/2 mM NaHCO3 and 60 mM NaNO3 (neutral salt) to measure enzyme activity. The specific activity of ATPase was estimated from the increase in the content of inorganic phosphorus (Pi) in 0.5 ml incubation medium at 30˚C for 30 min. Phosphorus concentration in samples was measured by the method of Chen and expressed in μmol Pi/h/mg protein [16] [17] . The activity of the “basal” Mg2+-ATPase was calculated as the difference between the ATPase activities in the presence and absence of MgSO4 in the incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM Tris-ATP and 60 mM NaNO3. The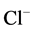 ,
, 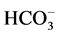 -activated Mg2+-ATPase was determined in the presence of
-activated Mg2+-ATPase was determined in the presence of 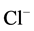 /
/ ions in the incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM Tris-ATP, 10 mM NaCl/2 mM NaHCO3 and 60 mM NaNO3. The enzyme activation by anions was calculated as the difference between the “basal” Mg2+-ATPase activities in the presence and absence of anions (chloride/bicarbonate) in the incubation medium. The figures show values of the enzyme activity averaged from the results of at least four determinations.
ions in the incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM Tris-ATP, 10 mM NaCl/2 mM NaHCO3 and 60 mM NaNO3. The enzyme activation by anions was calculated as the difference between the “basal” Mg2+-ATPase activities in the presence and absence of anions (chloride/bicarbonate) in the incubation medium. The figures show values of the enzyme activity averaged from the results of at least four determinations.
2.4. Assay of the Action of Chemicals on the ATPase Activity
The enzyme activity in the presence of chemicals (Ca2+, EGTA, GABA, bicuculline, picrotoxine, o-vanadate, genistein) was determined as described before [3] . Membrane samples were preincubated at 30˚C for 20 min with the relevant chemical in incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 10 mM NaCl/2 mM NaHCO3 and 60 mM NaNO3. The reaction was started by addition of the substrate (Mg2+-ATP) to the incubation medium.
2.5. Chemicals
All drugs were prepared as stock solutions in water unless otherwise stated. GABA, picrotoxin, bicuculline methochloride, CaCl2, EGTA, Tris, Hepes, Na2ATP, o-vanadate, genistein were by Sigma-Aldrich.
2.6. Statistics
The data are expressed with mean ± standard error where appropriate. The experimental data are statistically processed using one-way ANOVA test program “Statistica 7.0”. Evaluation of the significance of differences was carried out at p < 0.05 (n = 4).
3.1. Detection and Ca2+ Effect on the ,
,  -ATPase Activity
-ATPase Activity
We showed earlier that the multifunctional ATPase complex is the enzyme system, including “basal” Mg2+- ATPase, which is stimulated by anions and regulated (activated/inhibited) by GABAА-ergic ligands. In the samples of plasma membrane from rat brain studied by us, the activity of the “basal” Mg2+-ATPase is 8.8 μmol Pi/h/mg protein. This ATPase activity is stimulated by ions 10 mM Cl−/2 mM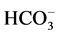 , the stimulation effect (
, the stimulation effect (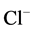 ,
,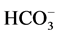 -ATPase activity) is 2.4 μmol Pi/h/mg protein.
-ATPase activity) is 2.4 μmol Pi/h/mg protein.
To verify that the enzymatic activity under study is a GABAA-coupled ATPase, we added GABAA-ergic ligands (GABA, bicuculline, picrotoxin) to the incubation medium. GABA (10 mM) activated the “basal” Mg2+- ATPase, while no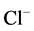 ,
, 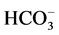 -ATPase activity could be detected. This effect of the mediator on the enzyme was eliminated by bicuculline (20 mM) and picrotoxin (50 mM) (Figure 1(a)). These data confirm that the ATPase activity under study belongs to the same enzyme—GABAA-coupled ATPase complex. Therefore, if in the presence of an activator the activity of the “basal” Mg2+-ATPase achieves high levels when the molecular turnover is maximal, then an additional activation of the enzyme by anions cannot take place.
-ATPase activity could be detected. This effect of the mediator on the enzyme was eliminated by bicuculline (20 mM) and picrotoxin (50 mM) (Figure 1(a)). These data confirm that the ATPase activity under study belongs to the same enzyme—GABAA-coupled ATPase complex. Therefore, if in the presence of an activator the activity of the “basal” Mg2+-ATPase achieves high levels when the molecular turnover is maximal, then an additional activation of the enzyme by anions cannot take place.
The literature shows that Ca2+ modulates the activity of the transport ATPase P-type of different cells. In particular, it was shown that EGTA (EDTA) and Ca2+ can modify the neuronal membrane Na+, K+-ATPase [19] [20] . At the same time, there was observed a change in the activity of both transport P-type ATPases and “total” Mg2+-ATPase or “basal” Mg2+-ATPase that are insensitive to ouabain [21] [22] . Therefore, we investigated the effect of Ca2+ on the “basal” and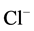 ,
, 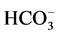 -activated Mg2+-ATPase activities of the neuronal membranes. In our experiments, Ca2+ (0.01 - 1 mM) was shown to reduce the activity of “basal” Mg2+-ATPase starting from the concentration of 0.05 mM (Figure 1(b)). In the highest studied concentration of Ca2+ (1 mM) the activity of the
-activated Mg2+-ATPase activities of the neuronal membranes. In our experiments, Ca2+ (0.01 - 1 mM) was shown to reduce the activity of “basal” Mg2+-ATPase starting from the concentration of 0.05 mM (Figure 1(b)). In the highest studied concentration of Ca2+ (1 mM) the activity of the

Figure 1. (a) The “basal” (I) and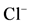 ,
, 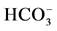 -stimulated (II) ATPase activities of rat brain plasma membranes in the absence (1) or in the presence of 10 mM GABA (2), 10 mM GABA + 20 mM bicuculline (3), 10 mM GABA + 50 mM pirotoxin (4) and (b) The “basal” (I) and
-stimulated (II) ATPase activities of rat brain plasma membranes in the absence (1) or in the presence of 10 mM GABA (2), 10 mM GABA + 20 mM bicuculline (3), 10 mM GABA + 50 mM pirotoxin (4) and (b) The “basal” (I) and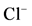 ,
,  -stimulated (II) Mg2+-ATPase activities in the presence of the different Ca2+ concentrations. Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
-stimulated (II) Mg2+-ATPase activities in the presence of the different Ca2+ concentrations. Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
“basal” Mg2+-ATPase decreased to 55% and amounted to 4.4 mmol Pi/h/mg protein. Along with this, the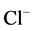 ,
, 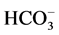 -ATPase activity doubled. In the presence of EGTA (0.1 mM), the activating effect of calcium ions on the enzyme activity does not occur (data not shown). Furthermore, this Ca2+ chelator causes an increase activity of “basal” Mg2+-ATPase by about 17%. These data indicate the presence of free calcium in the incubation medium similar to the literature data. So, in the presence of EDTA (40 mM) in the incubation medium, the activity of “basal” Mg2+-ATPase was increased by 65% [20] .
-ATPase activity doubled. In the presence of EGTA (0.1 mM), the activating effect of calcium ions on the enzyme activity does not occur (data not shown). Furthermore, this Ca2+ chelator causes an increase activity of “basal” Mg2+-ATPase by about 17%. These data indicate the presence of free calcium in the incubation medium similar to the literature data. So, in the presence of EDTA (40 mM) in the incubation medium, the activity of “basal” Mg2+-ATPase was increased by 65% [20] .
3.2. Effect of Ca2+ on the ATPase Activity in the Presence of GABAA-Ergic Drugs
The observed Ca2+ concentrations (0.5 - 1 mM) that cause the greatest change in the activity of the ATPase under study are similar to concentrations that inhibit the GABAA-induced 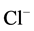 -current. It was shown that intracellular [Ca2+]i, depending on the concentration, has a multidirectional effect on the GABAA-receptors. Low (0.01 mM) concentrations of Ca2+ cause potentiation of the GABAA-induced
-current. It was shown that intracellular [Ca2+]i, depending on the concentration, has a multidirectional effect on the GABAA-receptors. Low (0.01 mM) concentrations of Ca2+ cause potentiation of the GABAA-induced 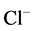 -current, while high (500 mM) concentrations reduce their functional activity by decreasing the opening time of the GABAA-receptor
-current, while high (500 mM) concentrations reduce their functional activity by decreasing the opening time of the GABAA-receptor 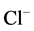 -channel [13] .
-channel [13] .
Furthermore, it was found that [Ca2+]i accelerates reduction of function (run-down effect) of GABAA-induced 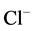 -current in hippocampal neurons in rats [23] [24] . Therefore, the next step in our work was to study the effect of Ca2+ on the GABAA-activated Mg2+-ATPase activity (Figure 2). We found that low concentrations of Са2+ (0.01 mM) do not affect this enzymatic activity. At the same time, high concentrations of Са2+ (0.25 mM) eliminate activation of the “basal” Mg2+-ATPase by GABA.
-current in hippocampal neurons in rats [23] [24] . Therefore, the next step in our work was to study the effect of Ca2+ on the GABAA-activated Mg2+-ATPase activity (Figure 2). We found that low concentrations of Са2+ (0.01 mM) do not affect this enzymatic activity. At the same time, high concentrations of Са2+ (0.25 mM) eliminate activation of the “basal” Mg2+-ATPase by GABA.
Bicuculline is known to competitively interact with the allosteric binding site close to the 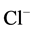 -channel of the GABAA/benzodiazepine receptor complex. This interaction results in a change in the conformation of
-channel of the GABAA/benzodiazepine receptor complex. This interaction results in a change in the conformation of 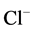 - channel and reduction of
- channel and reduction of  -conductance in the neuron [24] . The results of our earlier studies showed the functional and structural conjugation of the investigated neuronal membrane ATPase with GABAA/benzodiazepine
-conductance in the neuron [24] . The results of our earlier studies showed the functional and structural conjugation of the investigated neuronal membrane ATPase with GABAA/benzodiazepine
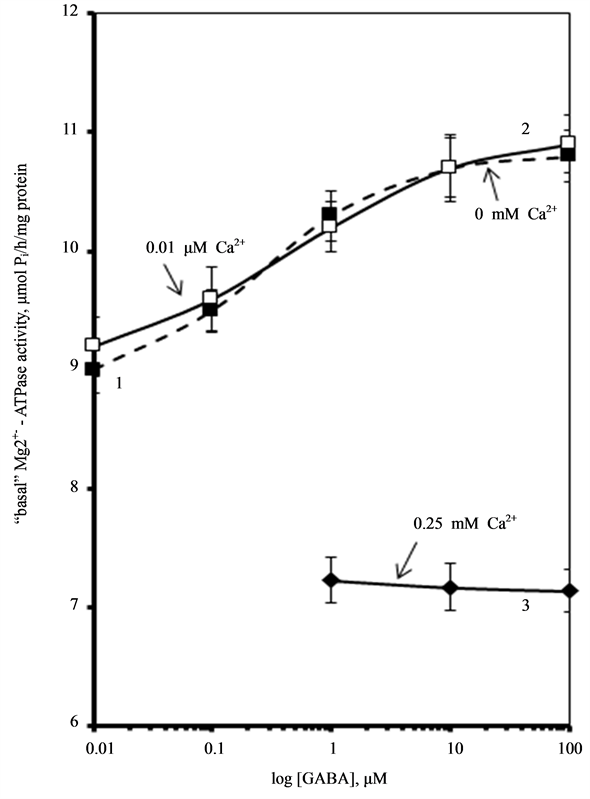
Figure 2. Effect of GABA on the “basal” Mg2+-ATPase activity of rat brain plasma membranes in the absence (1) and in the presence of 0.01 mM (2) or 0.25 mM (3) Ca2+ in the incubation medium. Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAA-ergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
 -channel receptor complex [18] . Therefore, we studied the effect of different concentrations (2.5 - 20 mM) of bicuculline on the
-channel receptor complex [18] . Therefore, we studied the effect of different concentrations (2.5 - 20 mM) of bicuculline on the ,
, 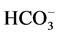 -ATPase activity in the absence and in the presence of Ca2+ (Figure 3(a)).
-ATPase activity in the absence and in the presence of Ca2+ (Figure 3(a)).
We have found that bicuculline inhibits the investigated activity starting with the concentration of 2.5 μM, and shows the greatest effect at the concentration of 15 μM. Ca2+ (0.25 mM) eliminates the inhibitory effect of bicuculline on the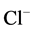 ,
,  -ATPase activity.
-ATPase activity.
These data suggest that Са2+ has protective properties against the action of the GABAA-receptor blocker on the enzyme. In this regard, it seemed appropriate to investigate the effect of different concentrations of Ca2+ (0.01 - 1 mM) on the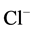 ,
, 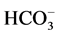 -ATPase activity in the presence of bicuculline (20 mM). It was found that Ca2+ eliminates the inhibitory effect of bicuculline on the ATPase activity starting from 50 μM (Figure 3(b)). The greatest increase of the effect of calcium cations is observed in the concentration range of 0.1 - 0.5 mM.
-ATPase activity in the presence of bicuculline (20 mM). It was found that Ca2+ eliminates the inhibitory effect of bicuculline on the ATPase activity starting from 50 μM (Figure 3(b)). The greatest increase of the effect of calcium cations is observed in the concentration range of 0.1 - 0.5 mM.
3.3. Role of Phosphatases on Modulation of the ATPase Activity by Ca2+
It is known from the literature that the function of the GABAA-receptors is supported by processes of
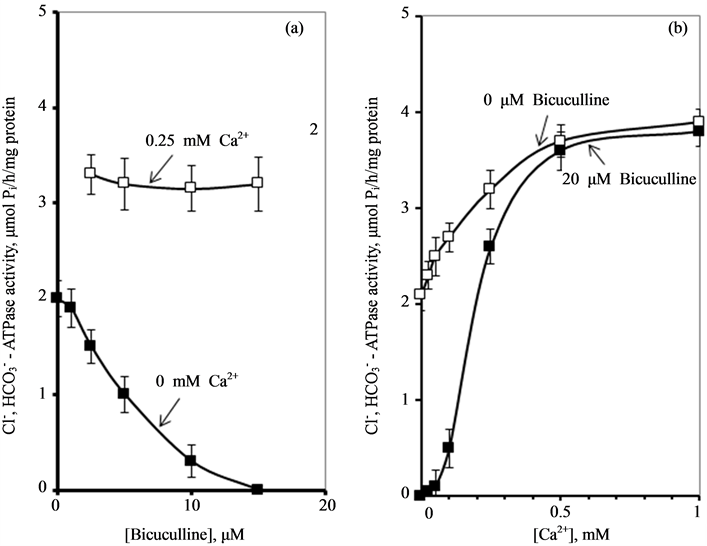
Figure 3. Effect of bicuculline (a) or calcium (b) on the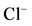 ,
, 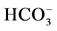 -stimulated ATPase activity of rat brain plasma membranes in the absence and in the presence of Ca2+ or bicuculline, respectively. Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
-stimulated ATPase activity of rat brain plasma membranes in the absence and in the presence of Ca2+ or bicuculline, respectively. Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
phosphorylation of receptor molecule or tightly bound regulatory molecules [13] [25] [26] . In the developed nervous system a high activity of protein tyrosine kinases and protein tyrosine phosphatases was shown, suggesting that protein tyrosine phosphorylation is an important factor for neuronal function. It has been shown that inhibitors of these enzymes regulate the functional activity of receptors involved in excitatory and inhibitory processes [10] . Thus, o-vanadate, blocker of protein phosphatases and transport ATPases P-type, increased the effect of GABA on the GABAA-receptors. Genistein and tyrphostin, blockers of protein tyrosine kinases, inhibited the GABAA-induced accumulation of 36Cl by brain membrane vesicles in mice, and GABAA-induced Cl--current in brain neuronal membranes in rats [27] [28] . These data suggest an important role of these enzymes in the maintenance of GABAA-receptor function. In our study, o-vanadate (0.1 mM) reduces the GABAA-induced Mg2+-ATPase activity. At the same time, genistein (0.1 mM) has no effect on this ATPase activity.
To investigate the possible involvement of these phosphatases in the action of Ca2+ on the investigated ATPase, we added o-vanadate and Ca2+ to the incubation medium (Figure 4). It was found that independent action of each of the two substances reduces the “basal” Mg2+-ATPase and increases the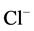 ,
, 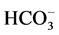 -ATPase activity. The combined action of these substances does not result in the increase of their inhibitory effect on the “basal” Mg2+-ATPase activity. These results are in a good agreement with the data obtained in the study of vanadatesensitive alkaline phosphatase conjugated with GABAA-receptors [13] . Its role in the regulation of the GABAA- receptor function was confirmed by addition of the enzyme to intracellular perfusate, which caused complete decline (run-down effect) of their function. Inhibition of such phosphatase by o-vanadate induced recovery of the GABAA-receptor function [10] [27] [28] .
-ATPase activity. The combined action of these substances does not result in the increase of their inhibitory effect on the “basal” Mg2+-ATPase activity. These results are in a good agreement with the data obtained in the study of vanadatesensitive alkaline phosphatase conjugated with GABAA-receptors [13] . Its role in the regulation of the GABAA- receptor function was confirmed by addition of the enzyme to intracellular perfusate, which caused complete decline (run-down effect) of their function. Inhibition of such phosphatase by o-vanadate induced recovery of the GABAA-receptor function [10] [27] [28] .
Previously, Hyden and colleagues showed the existence on rabbit Deiters’ neuron membrane of molecular (protein) machineries which recognize intracellular GABA and extrude chloride [29] . It was suggested that these structures are devices that at the expense of ATP consumed in their phosphorylation, extrude 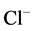 after postsynaptic GABA uptake into the Deiters’ neurons. The GABA effect was blocked by classical GABAA antagonists picrotoxin (100 µM) and bicuculline (10 µM) and also activated in a biphasic manner by pentobarbitone. Such
after postsynaptic GABA uptake into the Deiters’ neurons. The GABA effect was blocked by classical GABAA antagonists picrotoxin (100 µM) and bicuculline (10 µM) and also activated in a biphasic manner by pentobarbitone. Such
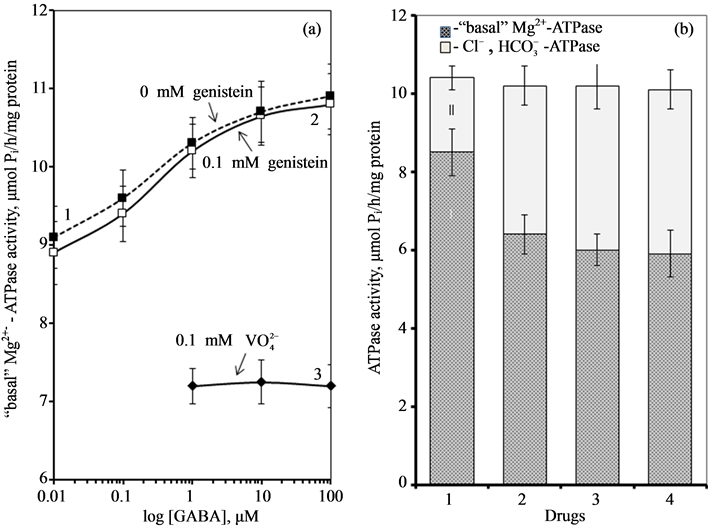
Figure 4. (a) Effect of GABA on the “basal» Mg2+-ATPase activity of rat brain plasma membranes in the absence (1) and in the presence of 0.1 mM genistein (2) or 0.1 mM o-vanadate (3); (b) The “basal” (I) and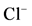 ,
, 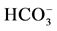 -stimulated (II) ATPase activities of the neuronal membrane in the absence (1) and in the presence 0.25 mM Ca2+ (2), 0.1 mM o-vanadate (3) and 0.25 mM Ca2+ + 0.1 mM o-vanadate (4). Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
-stimulated (II) ATPase activities of the neuronal membrane in the absence (1) and in the presence 0.25 mM Ca2+ (2), 0.1 mM o-vanadate (3) and 0.25 mM Ca2+ + 0.1 mM o-vanadate (4). Plasma membrane samples (20 - 25 mg) were added to incubation medium containing 12.5 mM HEPES-Tris (pH 7.4) and GABAAergic drugs and preincubated at 30˚C for 20 min. The reaction was started by addition of substrate (Mg2+-ATP) in the incubation medium.
properties have suggested to these authors that these receptors are GABAA-activated 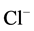 -pumps, where the energy for chloride extrusion is provided by ATP in a phosphorylation step within the extrusion cycle. The core mechanism is the inversion of two energy peaks along the permeation pathway. However, the role of ATP in the phosphorylation step of GABAA-regulated
-pumps, where the energy for chloride extrusion is provided by ATP in a phosphorylation step within the extrusion cycle. The core mechanism is the inversion of two energy peaks along the permeation pathway. However, the role of ATP in the phosphorylation step of GABAA-regulated 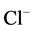 -pump is not conclusively established.
-pump is not conclusively established.
Analogously, the results of this work and preliminary our studies have shown that the investigated ATPase was inhibited by picrotoxin (or bicuculline) and regulated by modulators (anticonvulsants, benzodiazepines, anesthetics) [18] . Our biochemical and cytochemical findings enabled us postulating a new model of activity of the multifunctional ATPase complex—an enzyme that is also a 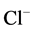 -pump and a receptor. We propose that the ATPase complex is closely related to GABAA-receptor and therefore can exist either in a phosphorylated ATPase complex-P (first functional state) or dephosphorylated form (second functional state). The former case has a low “basal” Mg2+-ATPase activity and it is activated by
-pump and a receptor. We propose that the ATPase complex is closely related to GABAA-receptor and therefore can exist either in a phosphorylated ATPase complex-P (first functional state) or dephosphorylated form (second functional state). The former case has a low “basal” Mg2+-ATPase activity and it is activated by 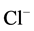 /
/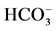 ions. This state enables the protein to participate in the ATP-dependent transport of anions. Phosphorylation is opposed by a dephosphorylation process which renders the ATPase complex as nonfunctional (the enzyme cannot participate in the ATP-dependent transport of anions). In this case, it has a high “basal” Mg2+-ATPase activity and it is not activated by anions. The dephosphorylation process is catalyzed by a vanadate-sensitive phosphatase. Thus, with the provision of Mg2+-ATP, a protein kinase (or directly ATP) phosphorylates the molecular complex and maintains the ATPase functional form. A similar cycle has been suggested to play a role in the regulation of GABAA-receptor [28] and GABAA-activated
ions. This state enables the protein to participate in the ATP-dependent transport of anions. Phosphorylation is opposed by a dephosphorylation process which renders the ATPase complex as nonfunctional (the enzyme cannot participate in the ATP-dependent transport of anions). In this case, it has a high “basal” Mg2+-ATPase activity and it is not activated by anions. The dephosphorylation process is catalyzed by a vanadate-sensitive phosphatase. Thus, with the provision of Mg2+-ATP, a protein kinase (or directly ATP) phosphorylates the molecular complex and maintains the ATPase functional form. A similar cycle has been suggested to play a role in the regulation of GABAA-receptor [28] and GABAA-activated 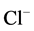 -pump. Yet, our results demonstrates that after convulsant effect, the dephosphorylation of the enzyme also occurs. As a result the ATPase doesn’t participate in the chloride transport (as the “collapsed” state). Our results suggest that not only inhibitors (o-vanadate, genistein, convulsants) have effect on the phosphatase activity, but also Ca2+ ions have an influence on the enzyme activity, as a result of their effect on the state of the protein phosphorylation.
-pump. Yet, our results demonstrates that after convulsant effect, the dephosphorylation of the enzyme also occurs. As a result the ATPase doesn’t participate in the chloride transport (as the “collapsed” state). Our results suggest that not only inhibitors (o-vanadate, genistein, convulsants) have effect on the phosphatase activity, but also Ca2+ ions have an influence on the enzyme activity, as a result of their effect on the state of the protein phosphorylation.
Cations of calcium play a vital role in the function of cells of various origin (including neurons). The concentration gradient of calcium across the plasma membrane of neuronal cells is a very high, from ~10−3 M Ca2+ outside, to ~10−7 M Ca2+ inside [30] . The free calcium concentration in neurons is supported by various mechanisms (buffering systems, compartmentation and extrusion from the neuronal cells). In earlier studies it was shown that Ca2+ (1 - 5 mM) decreased the number of GABA binding sites in rat cortical synaptic membranes [14] . Increases in [Ca2+]i (>1 µM) were reported in some works to reduce the open time [31] or to cause depression [23] of GABAA-activated 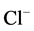 -channels in pituitary cells and dentate granule cells, respectively. In contrast, maintenance of a low level of [Ca2+]i (<0.1 µM) was required for full activation of GABAA-induced
-channels in pituitary cells and dentate granule cells, respectively. In contrast, maintenance of a low level of [Ca2+]i (<0.1 µM) was required for full activation of GABAA-induced 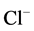 -current in guinea pig hippocampal neurons [32] . An increased intracellular Ca2+ concentration (10 nM - 34 µM) caused a transient augmentation of the GABAA-induced
-current in guinea pig hippocampal neurons [32] . An increased intracellular Ca2+ concentration (10 nM - 34 µM) caused a transient augmentation of the GABAA-induced 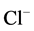 -current [15] . Moreover, it was established, that Ca2+ (depending on the concentration) has a biphasic effect on synaptic GABAA receptor
-current [15] . Moreover, it was established, that Ca2+ (depending on the concentration) has a biphasic effect on synaptic GABAA receptor 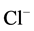 -channel [23] . So, the amplitude of GABAA-induced
-channel [23] . So, the amplitude of GABAA-induced 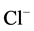 -current recorded with 1 mM internal CaCl2 and 10 mM EGTA (10 nM free Ca2+) decayed by less than 30% of control. At the same time, increasing the CaCl2 concentration to 10 mM (34 µM free Ca2+) induced a transient potentiation of the GABAA-current [33] .
-current recorded with 1 mM internal CaCl2 and 10 mM EGTA (10 nM free Ca2+) decayed by less than 30% of control. At the same time, increasing the CaCl2 concentration to 10 mM (34 µM free Ca2+) induced a transient potentiation of the GABAA-current [33] .
Calcium has been shown to exert a powerful inhibitory effect on the Na+, K+-ATPase of cell membranes [19] [34] . In particular, it was shown that in the presence of EDTA, Ca2+ (10−6 - 3 × 10−3 M) always exerts an inhibitory effect on the Na+, K+-ATPase [34] . Addition of Ca2+ to the incubation medium in the absence of EDTA caused no change in the “basal” Mg2+-ATPase activity at >10−3 M Ca2+. However at low (1 - 3 µM) Ca2+ in the media there was a significant stimulation of the Na+, K+-ATPase activity and decreasing as Ca2+ increased. So, at 10−3 M Ca2+ in the incubation medium an inhibition by 61% of the Na+, K+-ATPase activity occurred. Calcium concentrations that affect Na+, K+-ATPase are similar to concentrations that are effective on the ATPase we studied here. In our study, Ca2+ (>50 μM) inhibits the activity of basal Mg2+-ATPase and greatly increases the ,
, 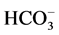 -ATPase activity. However the effects seen at millimolar Ca2+ levels may not be seen in the cell except, perhaps, transiently.
-ATPase activity. However the effects seen at millimolar Ca2+ levels may not be seen in the cell except, perhaps, transiently.
Our data demonstrate, for the first time, the sensitivity of the investigated multifunctional ATPase complex to calcium cations. This conclusion is well demonstrated by our results on reduction of the activity of “basal” Mg2+-ATPase and stimulation of the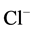 ,
, 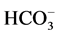 -ATPase activity with the increase of Ca2+ concentration in the incubation medium. Interdependent multidirectional response of these two ATPase activities to change in concentration of Ca2+ confirms the conjugation between investigated enzymes, and their association to the same complex. Furthermore, it indicates their involvement in Ca2+-dependent processes. This is confirmed by the results of the study of the effect of Ca2+ on the “basal” and
-ATPase activity with the increase of Ca2+ concentration in the incubation medium. Interdependent multidirectional response of these two ATPase activities to change in concentration of Ca2+ confirms the conjugation between investigated enzymes, and their association to the same complex. Furthermore, it indicates their involvement in Ca2+-dependent processes. This is confirmed by the results of the study of the effect of Ca2+ on the “basal” and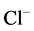 ,
, 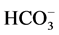 -activated Mg2+-ATPase activities in the presence of GABAA-ergic ligands, which are presented in this paper and in earlier studies. They also show a multidirectional GABAA-regulation of the ATPase activity. These data are in good agreement with the published data on the effect of Ca2+ on the functional activity of the GABAA-receptors [14] [15] . The authors demonstrated that GABAA-receptor activity varies depending on intracellular cation concentration. Moreover, Ca2+ can interact directly with the receptors either through binding sites on the molecule, or through receptor-conjugated enzyme systems (in particular Ca2+/calmodulin-dependent protein phosphatase, or via Ca2+-dependent protein kinase). In our study, the conjugation of the investigated ATPase activities and their involvement in Ca2+-dependent processes is also demonstrated by the effect of protein tyrosine phosphatase and protein tyrosine kinase blockers. Specifically, we have shown that the GABA-induced
-activated Mg2+-ATPase activities in the presence of GABAA-ergic ligands, which are presented in this paper and in earlier studies. They also show a multidirectional GABAA-regulation of the ATPase activity. These data are in good agreement with the published data on the effect of Ca2+ on the functional activity of the GABAA-receptors [14] [15] . The authors demonstrated that GABAA-receptor activity varies depending on intracellular cation concentration. Moreover, Ca2+ can interact directly with the receptors either through binding sites on the molecule, or through receptor-conjugated enzyme systems (in particular Ca2+/calmodulin-dependent protein phosphatase, or via Ca2+-dependent protein kinase). In our study, the conjugation of the investigated ATPase activities and their involvement in Ca2+-dependent processes is also demonstrated by the effect of protein tyrosine phosphatase and protein tyrosine kinase blockers. Specifically, we have shown that the GABA-induced 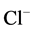 -ATPase activity is not sensitive to o-vanadate blocker but is inhibited by genistein blocker [35] . In contrast, in this work, o-vanadate in the absence of Ca2+ inhibited the GABAA-induced “basal” Mg2+-ATPase activity, and genistein did not affect the effect of the GABA on the enzyme. Therefore, although the “basal” and
-ATPase activity is not sensitive to o-vanadate blocker but is inhibited by genistein blocker [35] . In contrast, in this work, o-vanadate in the absence of Ca2+ inhibited the GABAA-induced “basal” Mg2+-ATPase activity, and genistein did not affect the effect of the GABA on the enzyme. Therefore, although the “basal” and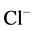 ,
, 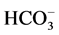 -activated Mg2+-ATPase activities are structurally conjugated, their functional activities can be dissociated by means of blockers of Ca2+-dependent protein kinases and protein phosphatases. This process can involve GABAAergic ligands.
-activated Mg2+-ATPase activities are structurally conjugated, their functional activities can be dissociated by means of blockers of Ca2+-dependent protein kinases and protein phosphatases. This process can involve GABAAergic ligands.
In this paper, we have found that in the presence of Са2+ in the incubation medium, no effect of bicuculline on the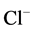 ,
, 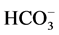 -ATPase activity could be observed. Based on these data, it can be concluded that Са2+ plays an ATPase-protective role. In therapeutic practice bicuculline is known as a convulsant. Therefore, our results can be important in the study of mechanisms of epileptogenesis, which are caused by various neurological or systemic disorders. This largely relates to electrolyte imbalance, especially calcium and magnesium imbalance. In particular, abnormalities of Са2+ homeostasis can affect the neuromuscular excitability, forming convulsive readiness in the brain, and hypocalcemia is often a side effect of anticonvulsant treatment [11] . In most cases, patients with epilepsy have hypocalcemia and hypomagnesemia [36] [37] . Our results on the protective role of Ca2+ for multifunctional ATPase during the action of convulsants suggest the use of these cations in the investigation of their anticonvulsive therapeutic effect in experiments in vivo.
-ATPase activity could be observed. Based on these data, it can be concluded that Са2+ plays an ATPase-protective role. In therapeutic practice bicuculline is known as a convulsant. Therefore, our results can be important in the study of mechanisms of epileptogenesis, which are caused by various neurological or systemic disorders. This largely relates to electrolyte imbalance, especially calcium and magnesium imbalance. In particular, abnormalities of Са2+ homeostasis can affect the neuromuscular excitability, forming convulsive readiness in the brain, and hypocalcemia is often a side effect of anticonvulsant treatment [11] . In most cases, patients with epilepsy have hypocalcemia and hypomagnesemia [36] [37] . Our results on the protective role of Ca2+ for multifunctional ATPase during the action of convulsants suggest the use of these cations in the investigation of their anticonvulsive therapeutic effect in experiments in vivo.
This study provides an additional biochemical characterization of multifunctional ATPase. Furthermore, cations of calcium regulate the ATPase activity in the absence of drugs. Consequently, the modulation of the multifunctional ATPase activity by GABAA-ergic drugs is a Ca2+-dependent process. It is important to understand the basic properties of this new multifunctional ATPase system and how it responds to changes in its environment. The obtained results seem to have an important functional significance in the study of the mechanisms of epileptogenesis and convulsant-induced seizure activity.
- Gerencser, G.A. and Zhang, J. L. (2003) Chloride ATPase Pumps in Nature: Do They Exist? Biological Reviews, 78, 197-218. http://dx.doi.org/10.1017/S146479310200605X
- Inagaki, C., Hara, M. and Zeng, X.T. (1996) A Cl−-Pump in Rat-Brain Neurons. Journal of Experimental Zoology, 275, 262-268.
- Menzikov S.A. and Menzikova, O.V. (2007) Comparative Properties of Sensitive to GABAA-Ergic Ligands, Cl−, HCO3−-Activated Mg2+-ATPase from Brain Plasma Membranes of Fish and Rats. Zhurnal Evoliutsionnoi Biokhimii Fiziologii, 43, 246-253.
- Bormann, J., Hamill, O.P. and Sakmann, B. (1987) Mechanism of Anion Permeation through Channels Gated by Glycine and Gamma-Aminobutyric Acid in Mouse Cultured Spinal Neurones. Journal of Physiology (London), 385, 243- 286.
- Menzikov S.A., Ruzhinskaia, N.N. and Menzikova, O.V. (2000) Mg2+-ATPase in the Fish Brain and Its Ultrastructural Localization. Zhurnal Evoliutsionnoi Biokhimii Fiziologii, 36, 263-267.
- Menzikov, S.A., Karpova, M.N. and Kalinina, M.V. (2011) Effect of HCO3− Ions on the ATP-Dependent GABAA Receptor-Coupled Cl− Channel in Rat Brain Plasma Membranes. Bulletin of Experimental Biology and Medicine, 152, 38-42. http://dx.doi.org/10.1007/s10517-011-1448-z
- Menzikov S.A. and Menzikova, O.V. (2006) Phosphorylation of Cl−, HCO3−-Stimulated Mg2+-ATPase of Plasma Membranes of Carp (Cyprinus carpio L.) Brain Sensitive to GABAA-Ergic Ligands. Ukrainskii Biokhimicheskii Zhurnal, 78, 63-69.
- Menzikov, S.A., Karpova, M.N. and Kalinina, M.V. (2012) Effect of Pentylenetetrazole on the GABAA-Coupled Cl−, HCO3−-Activated Mg2+-ATPase Activity of the Plasma Membrane from Rat Brain Both in Vitro and in Vivo Experiences. Pathological Physiology and Experimental Therapy, 98-102.
- Walton, N.Y., Nagy, A.K. and Treiman, D.M. (1998) Altered Residual ATP Content in Rat Brain Cortex Subcellular Fractions following Status Epilepticus Induced by Lithium and Pilocarpine. Journal of Molecular Neuroscience, 11, 233-242. http://dx.doi.org/10.1385/JMN:11:3:233
- Palma, E., Ragozzino, D.A., Angelantonio, S., Spinelli, Di G., Trettel, F., Martinez-Torres, A., Torchia, G., Arcella, A., Gennaro, G. Di., Quarato, P.P., Esposito, V., Cantore, G., Miledi, R. and Eusebi, F. (2004) Phosphatase Inhibitors Remove the Run-Down of Gamma-Aminobutyric Acid Type A Receptors in the Human Epileptic Brain. Proceedings of the National Academy of Sciences of the USA, 101, 10183-10188. http://dx.doi.org/10.1073/pnas.0403683101
- Kotova, S.M., Zasedateleva, I.Yu. and Koroleva, N.Yu. (2005) Peculiarities of Mineral Metabolism in Patients with Epilepsy. Journal of Neurology and Psychiatry (Russia), 3, 70-71.
- Сupello, A., Hyden, H., Rapallino, M.V. and Robello, M. (1998) Regulation by Intracellular Calcium of the Activity of GABAA Receptors in Two Different Types of Neurons. Neural Circuits and Networks NATO ASI Series, 167, 53-69.
- Chen, Q.X., Stelzer, A., Kay, A.R. and Wong, R.K. (1990) GABAA Receptor Function Is Regulated by Phosphorylation in Acutely Dissociated Guinea-Pig Hippocampal Neurons. The Journal of Physiology, 420, 207-221.
- Corda, M.G. and Guidotti, A. (1983) Modulation of GABA Receptor Binding by Ca2+. Journal of Neurochemistry, 41, 277-280. http://dx.doi.org/10.1111/j.1471-4159.1983.tb11840.x
- Aguayo, L.G., Espinoza, F., Kunos, G. and Satin, L.S. (1998) Effects of Intracellular Calcium on GABAA Receptors in Mouse Cortical Neurons. Pflugers Archiv, 435, 382-387. http://dx.doi.org/10.1007/s004240050527
- Chen, P.S., Toribara, T.Y. and Warner, H. (1990) Microdetermination of Phosphorus. Analytical Chemistry, 28, 1756- 1758. http://dx.doi.org/10.1021/ac60119a033
- Bradford, M.M. (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principal of Protein-Dye Binding. Analytical Biochemistry, 72, 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Menzikov S.A. (2013) Neuronal Multifunctional ATPase. Biophysical Reviews and Letters, 8, 213-227. http://dx.doi.org/10.1142/S1793048013300065
- Yingst, D.R. (1988) Modulation of the Na, K-ATPase by Ca and Intracellular Proteins. Annual Review of Physiology, 50, 291-303. http://dx.doi.org/10.1146/annurev.ph.50.030188.001451
- Powis, D.A. and Wattus, G.D. (1981) The Stimulatory Effect of Calcium on Na, K-ATPase of Nervous Tissue. FEBS Letters, 126, 285-288. http://dx.doi.org/10.1016/0014-5793(81)80262-1
- Tsakiris, S., Koromilas, C. and Schulpis, K.H. (2001) Reduced Mg2+-ATPase Activity in the Hypoglycemic Adult Rat Brain. Zeitschrift fur Naturforschung C-A. Journal of Biosciences, 56, 912-914.
- Vasic, V. Jovanović, D., Krstić, D., Nikezić, G., Horvat, A., Vujisić, L., et al. (1999) Prevention and Recovery of CuSO4-Induced Inhibition of Na+/K+-ATPase and Mg2+-ATPase in Rat Brain Synaptosomes by EDTA. Toxicology Letters, 110, 95-103. http://dx.doi.org/10.1016/S0378-4274(99)00144-7
- De Koninck, Y. and Mody, I. (1996) The Effects of Raising Intracellular Calcium on Synaptic GABAA ReceptorChannels. Neuropharmacology, 35, 1365-1374. http://dx.doi.org/10.1016/S0028-3908(96)00063-9
- Li, L., Wang, Y., Ma, K.T., Cheng, H.J., Zhao, L. and Si, J.Q. (2013) The Effect of Niflumic Acid and Blocker of Calcium Channel on the Desensitization of Gamma Aminobutyric Acid-Activated Current. Zhongguo Ying Yong Sheng Li Xue Za Zhi, 29, 128-132.
- Obrietan, K. and van den Pol, A.N. (1995) GABA Neurotransmission in the Hypothalamus: Developmental Reversal from Ca2+ Elevating to Depressing. The Journal of Neuroscience, 7, 5065-5077.
- Akopian, A., Gabriel, R. and Witkovsky, P. (1998) Calcium Released from Intracellular Stores Inhibits GABAA-Mediated Currents in Ganglion Cells of the Turtle Retina. Journal of Neurophysiology, 80, 1105-1115.
- Stelzer, A., Kay, A.R. and Wong, R.K. (1988) GABAA-Receptor Function in Hippocampal Cells Is Maintained by Phosphorylation Factors. Science, 241, 339-341. http://dx.doi.org/10.1126/science.2455347
- Jassar, B.S., Ostashewski, P.M. and Jhamandas, J.H. (1997) GABAA Receptor Modulation by Protein Tyrosine Kinase in the Rat Diagonal Band of Broca. Brain Research, 775, 127-133. http://dx.doi.org/10.1016/S0006-8993(97)00892-5
- Hydén H., Rapallino, M.V. and Cupello, A. (2000) Unraveling of Important Neurobiological Mechanisms by the Use of Pure, Fully Differentiated Neurons Obtained from Adult Animals. Progress in Neurobiology, 60, 471-499. http://dx.doi.org/10.1016/S0301-0082(99)00035-0
- Simons, T.J. (1988) Calcium and Neuronal Function. Neurosurgical Review, 11, 119-129. http://dx.doi.org/10.1007/BF01794675
- Taleb, O., Trouslard. J., Demeneix, B.A., Feltz, P., Bossu, J.L., Dupont, J.L. and Feltz, A. (1987) Spontaneous and GABA-Evoked Chloride Channels on Pituitary Intermediate Lobe Cells and Their Internal Ca Requirements. Pflügers Archiv, 409, 620-631. http://dx.doi.org/10.1007/BF00584663
- Stelzer, A., and Wong, R.K. (1987) GABA Receptors of Isolated Guinea-Pig Hippocampal Neurons: Intraand Extracellular Regulatory Factors. The Journal of Physiology (London), 394, 115-120.
- Tapia, J.C., Espinoza, F. and Aguayo, L.G. (1997) Differential Intracellular Regulation of Cortical GABAA and Spinal Glycine Receptors in Cultured Neurons. Brain Research, 769, 203-210. http://dx.doi.org/10.1016/S0006-8993(97)00672-0
- Abashidze, S., Jariashvili, T. and Kometiani, Z. (2001) The Effect of EGTA and Ca++ in Regulation of the Brain Na/KATP-ase by Noradrenaline. BMC Biochemistry, 2, 8. http://dx.doi.org/10.1186/1471-2091-2-8
- Menzikov S.A. and Menzikova, O.V. (2002) Effects of Orthovanadate and Genistein on the Plasma Membrane Cl−- ATPase Sensitive to GABAA-Ergic Ligands in the Bream (Abramis brama L.) Brain. Doklady Biological Sciences, 385, 334-336. http://dx.doi.org/10.1023/A:1019952515746
- Farhat, G., Yamout, B., Mikati, M.A., Demirjian, S., Sawaya, R. and Fuleihan, G.E.H. (2002) Effect of Antiepileptic Drugs on Bone Density in Ambulatory Patients. Neurology, 58, 1348-1353. http://dx.doi.org/10.1212/WNL.58.9.1348

*Corresponding author.
上一篇:Dexamethasone treatment alters 下一篇:Enzymes of Entomopathogenic Fu
